·Basic Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Safety of intravitreal quinupristin/dalfopristin in an
animal model
Veronica E.
Giordano1, Sergio E. Hernandez-Da Mota2, Tania N.
Adabache-Guel1, Armando Castillejos-Chevez3, Sonia
Corredor-Casas4, Samantha M. Salinas-Longoria1, Rafael Romero-Vera1, Juan M.
Jimenez-Sierra1, Jose L. Guerrero-Naranjo1, Virgilio
Morales-Canton1
1Department of
Ophthalmology, Retina and Vitreous Service, Asociacion para Evitar la Ceguera
en Mexico, Vicente García Torres 46, Coyoacán, Barrio San Lucas, Mexico City 04030, Mexico
2Ophthalmology Service, Clinica
David, Boulevard García de León 598, Nueva Chapultepec,
Morelia, Michoacán 58280, Mexico
3Department of Ophthalmology, Glaucoma Service,
Asociacion para Evitar la Ceguera en Mexico, Vicente García Torres 46,
Coyoacán, Barrio San Lucas, Mexico City 04030, Mexico
4Department of Ophthalmology, Pathology Service,
Asociacion para Evitar la Ceguera en Mexico, Vicente García Torres 46,
Coyoacán, Barrio San Lucas, Mexico City 04030, Mexico
Correspondence
to: Veronica E. Giordano. Division del Norte 2743 A 303, San Lucas 04030, Coyoacán, Distrito Federal,
Mexico. pinigiordano@hotmail.com
Received: 2015-01-10 Accepted: 2015-07-29
Abstract
AIM:
To determine whether different intravitreal doses of quinupristin/dalfopristin
lead to electroretinographic or histological changes in the rabbit retina over
one month period after injection.
METHODS:
Eighteen New Zealand white rabbits were divided into three treatment groups
(groups 1 to 3) and different intravitreal doses of quinupristin/dalfopristin
were tested in each group. The right eye was injected with the drug and the
left eye received intravitreal injection of 5% dextrose water and served as
control eye. The doses delivered to each group were 0.1 mg/0.1
mL, 1 mg/0.1 mL
and 10 mg/0.1 mL. Simultaneous, bilateral, dark-adapted electroretinography and
clinical images of both eyes were obtained in all groups before injection
(baseline) and after 7, 14, 21 and 28d, followed by enucleation for
histological examination.
RESULTS:
Subjects in the group 1 showed no signs of toxicity in the electroretinogram
when compared with groups 2 and 3 (Kruskall-Wallis test, P=0.000). By day 7, no electrical response to light stimuli was
recorded in the treated eyes in groups 2 and 3, consistent with severe damage
due to retinal toxicity. Light microscopy revealed no significant
histopathological changes in the group 1, while rabbits in groups 2 and 3 had
signs of granulomatous inflammation in most cases.
CONCLUSION: Intravitreal
0.1 mg/0.1 mL doses of quinupristin/dalfopristin do not lead to electroretinographic or histological
signs of retinal toxicity compared with 1 mg/0.1 mL and 10 mg/0.1 mL in this
rabbit model.
KEYWORDS:
endophthalmitis; quinupristin/dalfopristin; retinal toxicity
DOI:10.18240/ijo.2016.03.08
Citation:
Giordano VE, Hernandez-Da Mota SE, Adabache-Guel TN, Castillejos-Chevez A,
Corredor-Casas S, Salinas-Longoria SM, Romero-Vera R, Jimenez-Sierra JM,
Guerrero-Naranjo JL, Morales-Canton V. Safety of intravitreal quinupristin/dalfopristin
in an animal model. Int J Ophthalmol 2016;9(3):373-378
INTRODUCTION
Acute endophthalmitis is one of the most challenging
complications in ophthalmic surgery and portends a poor visual outcome. Because of the difficulty in obtaining
effective antibiotic levels within the eye via
parenteral or oral drug administration, intravitreal injection still remains
the mainstay of therapy. Because of the current high prevalence of infections
caused by Staphylococcus sp., the
treatment of choice is vancomycin and ceftazidime in order to cover Gram-negative bacteria; these are administered with or
without steroids[1]. However, in many cases, this treatment
is no longer effective particularly as a result of the increasing prevalence of
resistant bacteria found in clinical practice.
Quinupristin/Dalfopristin (Q-D) (Synercid, DSM
Pharmaceuticals, Inc., Greenville, NC, USA) is a novel drug that combines two streptogramins:
quinupristin (a B streptogramin) and dalfopristin (an A streptogramin) in a
30:70 ratio; it is indicated in the treatment of serious infections caused by
multiresistant Gram-positive organisms and exhibits extended activity against
vancomycin-resistant strains of staphylococci[2-4].
Q-D has a minimum inhibitory concentration (MIC) ≤ 1 µg/mL in
90% of Gram-positive isolates resistant to other drugs, including Staphylococcus aureus and Enterococcus faecium and a prolonged
antibiotic effect (up to 10h)[2,4].
Q-D has also
demonstrated in vitro inhibitory
activity of proinflammatory mediators, thus possibly affecting immunomodulatory
activity[5].
There are a few case reports in the literature in
which the use of intravitreal Q-D has resulted in a favorable outcome without
side ocular effects[5-6].
However to date, there are no published data on retinal toxicity of
intravitreal Q-D using histopathology or electroretinographic studies.
The purpose of this study is to determine the safety
of different Q-D doses administered in the vitreous of rabbit eyes.
MATERIALS AND METHODS
All experimental procedures in this study comply with
the statutes for care and handling of animals of the Association for Research
in Vision and Ophthalmology (ARVO); all care, production and experimental animal
use followed the official Mexican standard NOM-062-ZOO-1999 guidelines;
biological waste was disposed of in compliance with the standard
NOM-087-ECOL-94 laws. The study protocol was approved by the Ethics Committee
and Review Board of the Association for Prevention of Blindness Hospital,
Mexico City, Mexico.
Eighteen New Zealand white rabbits (weighing
approximately 2500 g each) were used and randomly assigned to study groups 1,
2, and 3 (six eyes per treatment group). The 18 eyes were randomly distributed
to receive 1 of 3 different intravitreal doses in the right eye: group 1
received 0.1 mg/0.1 mL; group 2 received 1 mg/0.1 mL and group 3 received
10 mg/0.1 mL. In every subject, the right eye received the Q-D injection, while
the left eye served as its control.
The rabbits were
sedated with an intravenous ketamine dose of 10 mg/kg (King Pharmaceutical,
Inc., Bristol, TN, USA) and a topical dose of 5 mg/mL of proparacaine (Sophia Laboratories,
Inc., México City, TX, USA). After sedation, 0.1 mL of the
corresponding concentration of Q-D was injected into the right eye, and left
eye received intravitreal injection of 5% dextrose water and served as control
eye. Intravitreal injection was performed under sterile conditions, using a 27
gauge needle, in the temporal sclera as the injection site.
Solution Preparation and Administration A 500 mg
single dose Q-D vial was reconstituted under aseptic conditions under a laminar
air flow hood, by slowly adding 5 mL of 5% dextrose in water. The vial was then
manually stirred by rotational movements to avoid foam formation. The resultant
concentration of the Q-D solution was 100 mg/mL.
The reconstituted solution was diluted again within
30min in 5% dextrose solution to obtain the appropriate concentrations assigned
to each group (group 1: 0.1 mg/0.1 mL; group 2: 1 mg/0.1 mL and group 3: 10 mg/0.1 mL) for injection.
As indicated by
the manufacturer, injections were applied at room temperature and within 1h of
preparation to ensure stability of the drug.
Treatment was
administered-slowly and under direct visualization-in the mid-vitreous of each eye, with the bevel of the
needle positioned upwards.
After 28d, all rabbits were euthanized with an
overdose of intravenous sodium pentobarbital (0.36 mg/kg) and the eyes were
enucleated and stored in 15 mL 10% formalin until histological preparation.
Ophthalmoscopic Studies
All eyes were examined on the day before treatment
(day 0) and on days 7, 14, 21 and 28. Thirty minutes before examination, the
pupils were dilated with 2 drops of 0.5% tropicamide and 15min later, with 0.5%
phenylephrine hydrochloride. The eyes were examined by indirect ophthalmoscopy
and were photographed (FF 450 plus, IR, AVTZK5, Carl Zeiss, Germany).
Electroretinography
Simultaneous bilateral electroretinography
(ERG) was performed prior to injection and 1, 2, 3 and 4wk after injection in
all 18 rabbits.
Under a dim red light, the rabbits were anesthetized
and one drop of topical anesthesia was applied in each eye. The pupils were
dilated with 2 drops of 0.5% tropicamide and 0.5% phenylephrine hydrochloride
15min later, 30min before the study. Two recording electrodes (JET; LKC
Technology, Gaithersburg, MD, USA) were placed in each eye via contact lenses and a ground electrode was placed on the forehead.
The skin of the forehead had been previously shaved and cleaned, and a
conductive cream was applied prior to electrode placement. Impedance was set to
less than 5 ω in each electrode. The animals were adapted to the
dark for 20min. After anesthesia induction, electrode placement and ERG
recordings were performed under dim red light. White flashes to determine
corneal electrical responses were delivered with a full-field Ganzfeld stimulator and Nicolet Ganzfeld amplifier (Nicolet, Madison, Wisconsin, USA); responses were measured and recorded in mesopic conditions. The
a-wave and the b-wave were measured in all subjects. In compliance with the
International Society for Clinical Electrophysiology (ISCEV) guidelines, the
a-wave amplitude was measured from baseline to the a-wave’s trough and the
b-wave’s amplitude was measured from the a-wave trough to the b-wave peak[7].
A and b waves
were measured in the scotopic 3.0 ERG phase.
Euthanization and Histological Study Animals
were euthanized with an intravenous overdose of sodium pentobarbital (0.36
mg/kg). The
animal’s death was determined with the heart rate, respiratory rate and
response to stimuli. Once the procedure was completed, the animal’s eyes were
enucleated and fixed in 10%
neutral-buffered formaldehyde.
The globes were dissected horizontally and the
calottes were processed and embedded in paraffin. Five-micrometer sections of
the bisected globe were cut and evaluated by a pathologist unaware of the
study’s protocol. The anterior and posterior segments of the vitreous, nerve
fiber layer, retinal ganglion cell layer, bipolar cell layer, photoreceptor
layer, retinal pigment epithelium and choroid were evaluated for toxicity. For
the sake of consistency, the same pathologist randomly reevaluated 25 slides.
The vitreous was directly examined in the histological
slides and graded according to the presence of vitreous-retinal fibrovascular
membranes as follows: Grade 0: absent membranes; Grade 1: membranes present in
less than 25% of 10× power fields; Grade 2: membranes present in 25%-50% of 10× power fields; Grade 3: membranes present in 50%-75% of 10× power fields; Grade 4: membranes present in more than
75% of 10× power fields.
Retinal degenerative changes were graded as follows:
Grade 0: the retina maintained its normal histological appearance and a normal
number of ganglion cells; Grade 1: focal loss of histological architecture and
partial loss of ganglion cells; Grade 2: loss of most ganglion cells and
sectional loss of histological architecture; Grade 3: absence of ganglion cells
and widespread loss of histological architecture-some remaining clumps of nuclear layers may be
recognized.
The following observations were recorded: choroidal
changes, congestion (due to optic nerve compression during enucleation),
inflammation (lymphocytes), vacuolated histiocytes, foreign body giant cells
and calcium deposition.
Statistical Analysis
Data were analyzed with SPSS, version 20 for Mac; (SAS
Institute Inc., Cary, NC, USA). Kruskall-Wallis test was used for intergroup
analysis of the mean a and b wave amplitude values in the scotopic 3.0 ERG
(maximal response phase). Friedman test was used for intragroup analysis.
Mann-Whitney test was used for post-hoc comparisons.
For all
analyses, a 2-sided P<0.05 was considered statistically significant.
RESULTS
Ophthalmoscopic Examination and Clinical
Pictures All eyes were free of cataracts, vitreous opacities or
bands at baseline examination (day 0). There were no significant differences between the
injected and control eye in the group 1 throughout follow-up (Figure 1).
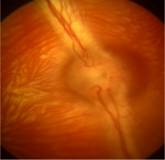
Figure 1 Retinal fundus photograph of an injected eye of group 1 Optic nerve and vessels are normal appearance
21d after
injection (0.1 mg/0.1 mL).
Vitreous opacities or band formation were not evident
in any of the control eyes in all groups. In the treated eye in groups 2 and 3
(1 mg/0.1 mL and 10 mg/0.1 mL doses), vitreous opacities, various degrees of
vitreous hemorrhage, retinal hemorrhages and vitreous bands were evident on the
first post-injection examination, and on day 7 (Figure 2).
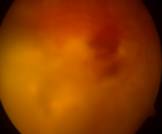
Figure 2 Retinal fundus photograph of an injected eye of group 2 Retinal fundus photograph of an injected eye of group
2: 1 mg/0.1 mL, showing vitreous and retinal hemorrhages 21d after injection.
Electroretinography A total of 18 injected and 18 control eyes were analyzed.
All subjects in the group 1 showed no signs of toxicity in the ERG when
compared with control eyes. In the group 1, the a wave and b wave amplitudes in
the injected eyes were stable, with no significant variation 4wk after
injection (Figure 3).

Figure 3 The a-wave amplitude in the 3.0 scotopic ERG of one injected eye of group 1 A: Scotopic ERG of group 1 in day 0; B :No significant variation at 4 wk after injection.
Sensitivity and sweep time per division: 100 μV and 20ms.
All treated eyes in groups 2 and 3 showed no
electrical response to light stimuli in the 3.0 scotopic ERG, 1wk after
injection, reflecting severe retinal toxicity (Figure 4).
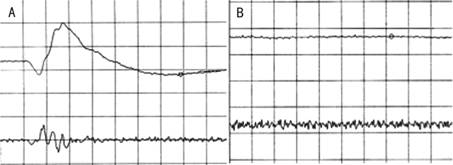
Figure 4 The a-wave and b-wave amplitudes in the 3.0 scotopic ERG of one injected eye of group 2 A: Scotopic ERG of one injected eye of group 2 in day 0;
B: Abolition to light stimuli at 1wk after injection. Sensitivity
and sweep time per division: 100 μV and 20ms.
All subjects in groups 2 and 3 were euthanized after
the first week and no further electrophysiological recordings were performed.
Subjects in the group 1 showed no signs of toxicity in
the ERG when compared with groups 2 and 3 (Friedman test, P=0.000). There was a statistically significant difference between
the three groups (P=0.000).
ERG results are
shown in Tables 1 and 2.
Table 1 Mean a-wave and b-wave amplitudes mV;
![]()
|
Wave amplitude |
Baseline |
Week
1 |
Week
2 |
Week
3 |
Week
4 |
P |
|
Mean a-wave amplitude |
|
|
|
|
|
|
|
Group 1 (0.1mg) |
46.8±37.8 |
68.7±16.7 |
53.1±9.4 |
45.8±17.5 |
43.7±17.2 |
0.373 |
|
Group 2 (1 mg) |
40.9±14.7 |
0 |
- |
- |
- |
0.000 |
|
Group 3 (10 mg) |
45.27±6.7 |
4.5±9.1 |
- |
- |
- |
0.006 |
|
Mean b-wave amplitude |
|
|
|
|
|
|
|
Group 1 (0.1 mg) |
169.7±44.8 |
242.7±25.1 |
185.4±37.6 |
175±44 |
152±17.9 |
0.010 |
|
Group 2 (1mg) |
109.9±45.1 |
0 |
- |
- |
- |
0.000 |
|
Group 3 (10 mg) |
143.1
±24.1 |
0 |
- |
- |
- |
0.000 |
Table 2 Comparisons of a-wave and b-wave amplitudes
between treated eyes and control eyes mV;
![]()
|
Wave amplitude |
Treated
eyes |
Control
eyes |
P |
|
Mean a-wave amplitude |
|
|
|
|
Group 1
(0.1mg) |
52.8±17.6 |
57.2±24.6 |
0.876 |
|
Group 2 (1mg) |
10.2±19.3 |
43.6±17.1 |
0.000 |
|
Group 3 (10
mg) |
9.9±18.7 |
55.6±18.6 |
0.000 |
|
Mean b-wave amplitude |
|
|
|
|
Group 1 (0.1
mg) |
188.8±45.8 |
167.1±39.9 |
0.072 |
|
Group 2 (1 mg) |
32.9±56.6 |
131±54.7 |
0.000 |
|
Group 3 (10
mg) |
33.1±60.5 |
129.7± 63.9 |
0.000 |
Histological Examination In a random and blinded manner, initial examination of
sections obtained in group 1 showed integrity of all retinal layers, normal
ganglion cell density and the presence of small caliber blood vessels in the
inner limiting membrane of the posterior pole (these vessels have been
described in the literature as normally present in the posterior pole retina of
healthy rabbits). There were no pathologic changes observed in the outer
retinal layers, nor in the retinal pigment epithelium. The anterior chamber and
vitreous showed no anomalies. The choroid had some degree of congestion in all
cases. Periodic acid-Schiff (PAS) and Gram stains were negative for microorganisms.
On the other hand, the eyes in groups 2 and 3 revealed
pathologic changes in the vast majority. Five of the ten eyes showed some
degree of congestion in the anterior segment. Vitreous examination revealed
Grade 3 vitreoretinal membranes (6 eyes), and Grade 4 in 1 eye; extracellular
foreign material vacuoles were observed in 5 eyes and histiocytic vacuoles with
intracellular foreign material in 3 eyes.
Close examination of the choroid showed congestion,
mild inflammation and vacuolated multinucleated giant cells in 7 eyes, 5 of
which also had intracellular calcium deposits. Only 3 of 10 eyes had mild choroid congestion.
All retinas in
groups 2 and 3 had Grade 3 degenerative changes.
PAS staining showed vacuoles in histiocytes, granular
material (calcium in some) in giant extracellular cells in 8 eyes and no
remarkable features were observed in the other 2 eyes.
Gram stain was
negative to microorganisms.
In summary, there were no significant
histopathological changes in the treated eyes of group 1 (Figure 5), while
signs of granulomatous inflammation were found in most of the eyes of groups 2
and 3 (Figure 6). Image taken with light microscopy at high
magnification with hematoxylin and eosin, showing the presence of calcium in
the thickness of the retina, signs of granulomatous inflammation, and the
presence of multinucleated giant cells of foreign body type, as well as
degenerative changes. Image taken with light microscopy at medium magnification
(10×) with hematoxylin and eosin illustrating retinal posterior pole with
recent major bleeding and granulomatous reaction around extensive calcium
deposition in the thickness of the retina with multiple degenerative changes.
Note the presence of vitreous membranes
(Figure 6).

B A
Figure 5 Histologic specimen of a treated eye of a
rabbit from group 1
Intermediate histological image magnification (10×) with hematoxylin and eosin
showing
artificially detached retina. There is normal integrity of all layers with
normal density of ganglion cells. No signs of inflammation nor vitreous
membranes were observed.

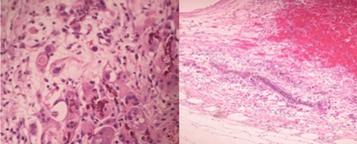
Figure 6 Histologic specimen of a treated eye of a rabbit from group 3 A: The presence of calcium in the retina, signs of
granulomatous inflammation, and the presence of multinucleated giant cells of
foreign body type; B: Retinal posterior pole with recent major bleeding and
granulomatous reaction around extensive calcium deposition.
DISCUSSION [Top]
The introduction of intravitreal antibiotics
revolutionized the treatment of infectious endophthalmitis, especially that due
to bacteria. Several intravitreal antibiotics have been studied and used.
Amikacin induces retinal toxicity as does intravitreal
trovafloxacin;
but others such as intravitreal garenoxacin appeared to be safe in an animal
model and, levofloxacin appears to be effective in treating experimental
endophthalmitis, but further studies are needed[8-12]. In a recent review, the susceptibility, in endophthalmitis samples of
bacterial isolates, to ceftazidime and vancomycin was studied and they conclude
that they still remain the therapy of choice for this entity[11]. Nevertheless, because of the current high prevalence of bacterial
resistance to vancomycin, and ceftazidime, treatment needs to be modified based
on clinical response.
Novel antibiotics such as moxifloxacin and
gatifloxacin, fourth-generation fluoroquinolones, have
enhanced activity against Gram-positive bacteria while retaining potent
activity against most Gram-negative bacteria, but intravitreal safety has not
been demonstrated, and bacterial resistance was demonstrated in ocular samples[13-15].
Although many eyes are successfully treated with the
use of intravitreal antibiotics-particularly when promptly administered-there are
still many cases that do not respond adequately and have a poor visual outcome;
this is partly due to the inflammatory phenomena occurring in endophthalmitis
that are not quelled by intravitreal antibiotics alone. This is why some
believe that the use of concomitant intravitreal steroids might be beneficial,
although this therapeutic approach is still somewhat controversial[16].
Q-D is a streptogramin antibiotic that aside from its
antibacterial properties. It has been shown to inhibit in vitro proinflammatory mediators such as IL-1α, IL-1β, IL-6 and TNF-α, suggesting a possible associated immunomodulatory
activity[5].
These properties
might be of some advantage when treating Gram-positive bacterial
endophthalmitis versus conventional intravitreal therapy.
There have been
a few reports of intravitreal Q-D in humans with bacterial endophthalmitis
resulting in favorable outcomes[5-6].
Hernandez-Da Mota[6]
reported a successful single case using a dose of 0.4 mg/0.1 mL and Stroh[5]
reported two successful cases of endophthalmitis caused by vancomycin-resistant
strains, treated with a similar dose of Q-D. This is consistent with
the findings in this study, where doses below 1 mg/0.1 mL did not lead to
significant toxic effects in the studied eyes.
ERG showed no statistically significant differences
between the amplitudes of the retinal a and b waves in rabbits injected with a
low dose of Q-D (group 1) nor in control eyes, 2 and 4wk after injection.
However, b wave
amplitude flattening and even a total loss of response were observed in groups
2 and 3, reflecting retinal toxicity. Also there was a more pronounced band
formation in groups 2 and 3, which might be a sign of inflammatory reponse as
well as toxicity.
Histological examination revealed no significant changes
using doses of 0.1 mg/0.1 mL; however, granulomatous reactions were observed in
the other two groups. These histopathological findings correlate with the
electrophysiology results in the groups on doses above 1 mg/0.1
mL.
Retinal toxicity profiles have been conducted with
other novel antibiotics. Kernt et al[14] reported that doses up
to 150 μg of moxifloxacin administered in the vitreous did not
damage different retinal cells. Aydin et
al[17] studying the intravitreal
toxicity of doxycycline, found that the group treated with the antibiotic exhibited
significant decreases in the ERG with doses ranging between 250 and 2000 μg per 0.1 mL. No significant changes in the ERG were
observed following the injection of lower doses[17]. Linezolid, a potent anti-staphylococcal
antibiotic, is safe at a dose of 30 mg after intravitreal administration[18]. Daptomycin
is reported to induce a total loss of the photoreceptor layer with doses of 750
μg, but doses up
to 188 μg did not induce deleterious changes in the retina[19]. Comer et al[19] reported that intravitreal daptomycin doses above
200 μg resulted in ERG abnormalities, while doses between 75 and 188 μg
did not lead to changes in the scotopic and photopic waves of the ERG; moderate depression was exhibited in the 375 μg
dose range, and severe depression resulted with the 750 μg
dose.
This study has numerous limitations that must be
considered. The small number of animals used (6 for each group) could mask a
possible real difference (type 2 statistical error). Since no statistically
significant difference in electroretinographic activity between Q-D and control
eyes was found in the low dose group, careful attention to the power of the
study is required, specially assuming that a clinically insignificant change in
electroretinographic activity would be less than a 20% difference between wave
amplitudes.
There are some issues that remain unsolved. It is
unknown whether doses between 0.1 mg/0.1
mL and 1 mg/0.1
mL are toxic.
This should be interesting since successfully treated cases of endophthalmitis
managed with Q-D fall within this dose range[5-6].
Further animal studies may be necessary to determine this factor. However,
based on these results and what has been previously reported, it seems that the
window of Q-D intraocular toxicity might not be as narrow as other antibiotics
that have been administered into the vitreous such as amikacin. Whether
these doses have an adequate antibacterial activity with the levels reached in
the vitreous cavity, retina and choroid also remains to be determined.
Further studies will also be needed on the Q-D minimum
inhibitory concentrations in vitro,
followed by animal induced endophthalmitis models to prove its safety and
efficacy as well as its pharmacokinetic characteristics.
Another question
arising with the use of Q-D in infectious endophthalmitis is whether combining
it with other antibiotics might provide additional benefits. This issue should
also be addressed in further studies since it has already been confirmed in
other systemic infections[4].
Comparative animal endophthalmitis model studies also need to be conducted with
standard intravitreal antibiotics such as vancomycin and ceftazidime. These
issues need to be addressed in order to further assess the role of intravitreal
Q-D in human bacterial endophthalmitis.
ACKNOWLEDGEMENTS [Top]
Conflicts of
Interest: Giordano VE,
None; Hernandez-Da Mota SE, None; Adabache-Guel
TN, None; Castillejos-Chevez A, None; Corredor-Casas S,
None; Salinas-Longoria SM, None; Romero-Vera
R, None; Jimenez-Sierra JM, None; Guerrero-Naranjo JL, None; Morales-Canton
V, None.
REFERENCES [Top]
1 Busbee BG. Advances in knowledge and treatment: an update on
endophthalmitis. <ii>Curr Opin Ophthalmol</ii> 2004;15(3):232-237.
[CrossRef]
2 Nailor MD,
Sobel JD. Antibiotics for gram-positive bacterial infections: vancomycin,
teicoplanin, quinupristin/dalfopristin, oxazolidinones, daptomycin,
dalbavancin, and telavancin. <ii>Infect Dis Clin North Am</ii>
2009;23(4):965-982. [CrossRef] [PubMed]
3 Finch RG.
Antibacterial activity of quinupristin/dalfopristin. Rationale for clinical
use. <ii>Drugs </ii> 1996;51 Suppl 1:31-37. [CrossRef] [PubMed]
4 Brown J,
Freeman BB 3rd. Combining quinupristin/dalfopristin with other agents for
resistant infections. <ii>Ann Pharmacother </ii> 2004;
38(4):677-685. [CrossRef] [PubMed]
5 Stroh EM.
Quinupristin/dalfopristin in vancomycin-resistant Staphylococcus aureus
endophthalmitis. <ii>Arch Ophthalmol</ii> 2012;130(10):1323-1324. [CrossRef] [PubMed]
6
Hernandez-Da Mota SE. Quinupristin/dalfopristin in Staphylococcus aureus
endophthalmitis: a case report<ii>. J Med Case Rep</ii> 2011;5:130.
[CrossRef]
[PubMed]
[PMC free article]
7 Marmor MF,
Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for
full-field clinical electroretinography (2008 update). <ii>Doc Ophtalmol
</ii> 2009;118(1):69-77. [CrossRef]
[PubMed]
8 Ferrer C,
Rodríguez A, Abad JL, Fernandez J, Alió JL. Bactericidal effect of intravitreal
levofloxacin in an experimental model of endophthalmitis. <ii>Br J
Ophthalmol</ii> 2008;92(5):678-682. [CrossRef] [PubMed]
9 Esfahani
MR, Kazi AA, Peyman GA, Dellacroce JT, Aydin E. Intravitreal toxicity of
garenoxacin. <ii>Retina</ii> 2006;26(2):182-186. [CrossRef]
10 Ng EW,
Joo MJ, Au Eong KG, Green WR, O'Brien TP. Ocular toxicity of intravitreal
trovafloxacin in the pigmented rabbit. <ii>Curr Eye Res</ii>
2003;27(6):387-393. [CrossRef]
11 Reddy AK,
Reddy RR, Paruvelli MR, <ii>et al</ii>. Susceptibility of bacterial
isolates to vancomycin and ceftazidime from patients with endophthalmitis: Is
there a need to change the empirical therapy in suspected bacterial
endophthalmitis? <ii>Int Ophthalmol</ii> 2014 Nov 11. [Epub ahead
of print].
12 Seawright
AA, Bourke RD, Cooling RJ. Macula toxicity after intravitreal amikacin.
<ii>Aust N Z J Ophthalmol </ii> 1996;24(2):143-146. [CrossRef]
13 Mehta S,
Armstrong BK, Kim SJ, Toma H, West JN, Yin H, Lu P, Wayman LL, Recchia FM,
Sternberg P Jr. Long-term potency, sterility, and stability of vancomycin,
ceftazidime, and moxifloxacin for treatment of bacterial endophthalmitis.
<ii>Retina </ii>2011;31(7):1316-1322. [CrossRef]
[PubMed]
14 Kernt M,
Neubauer AS, Ulbig MW, Kampik A, Welge-Lüssen U. <ii>In vitro</ii>
safety of intravitreal moxifloxacin for endophthalmitis treatment. <ii>J
Cataract Refract Surg</ii> 2008;34(3):480-488. [CrossRef]
[PubMed]
15 Wong CA,
Galvis V, Tello A, Villareal D, Rey JJ. In vitro antibiotic susceptibility to
fluoroquinolones. <ii>Arch Soc Esp Oftalmol</ii> 2012;87(3):72-78.
[CrossRef] [PubMed]
16 Albrecht
E, Richards JC, Pollock T, Cook C, Myers L. Adjunctive use of intravitreal
dexamethasone in presumed bacterial endophthalmitis: a randomised trial.
<ii>Br J Ophthalmol </ii> 2011;95(10):1385-1388. [CrossRef] [PubMed]
17 Aydin E,
Kazi AA, Peyman GA, Esfahani MR, Muñoz-Morales A, Kivilcim M, Caro-Magdaleno M.
Retinal toxicity of intravitreal doxycycline. A pilot study. <ii>Arch Soc
Esp Oftalmol</ii> 2007;82(4):223-228. [CrossRef] [PubMed]
18 Saleh M,
Lefèvre S, Acar N, Bourcier T, Marcellin L, Prévost G, Subilia A, Gaucher D,
Jehl F. Efficacy of intravitreal administrations of linezolid in an
experimental model of S. aureus-related endophthalmitis. <ii>Invest
Ophthalmol Vis Sci </ii> 2012;53(8):4832-4841. [CrossRef] [PubMed]
19 Comer GM,
Miller JB, Schneider EW, Khan NW, Reed DM, Elner VM, Zacks DN. Intravitreal
daptomycin: a safety and efficacy study. <ii>Retina </ii>
2011;31(6):1199-1206. [CrossRef] [PubMed] [PMC free article]
[Top]