·Clinical Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Serum
vascular endothelial growth factor receptor-2 and adropin levels in age-related
macular degeneration
Nurgül Örnek1,
Kemal Örnek1, Süleyman Aydin2, Musa Yilmaz2,
Yaşar Ölmez1
1Department
of Ophthalmology, School of Medicine, Kirikkale
University, Kirikkale 71450, Turkey
2Department
of Biochemistry, School of Medicine, Firat University, Elazig 23300, Turkey
Correspondence
to: Kemal Örnek. Department of
Ophthalmology, School of Medicine, Kirikkale University, Yahşihan, Kirikkale 71450,
Turkey. kemalornek@hotmail.com
Received:
2015-05-01
Accepted: 2015-07-20
Abstract
AIM:
To investigate the serum levels of vascular endothelial growth factor
receptor-2 (VEGFR-2) and adropin in age-related macular
degeneration (AMD) patients.
METHODS: Ninety-eight AMD patients were included
in the study. Seventy-eight age- and sex-matched healthy volunteers were
recruited as the control group. Fundus florescein angiography and optical
coherence tomography were performed to assess the posterior segment details.
Serum VEGFR-2 and adropin levels were measured using enzyme-linked
immunosorbent assays and compared between the study groups.
RESULTS: AMD group had
significantly increased foveal retinal thickness, serum LDL and HDL levels and
significantly decreased subfoveal choroidal thickness (P =0.01, 0.047, 0.025
and <0.001, respectively). Serum VEGFR-2 level revealed a significant
decrease in AMD patients compared to controls (26.48±6.44 vs 30.42±7.92 ng/mL, P<0.001).
There was an insignificant increase in serum adropin level in AMD patients
(6.17±3.19 vs 5.79±2.71 ng/mL, P=0.4) . Serum level of VEGFR-2 in AMD
patients had a significant negative correlation with foveal retinal thickness (r=-0.226,
P=0.025)
and a significant positive correlation with subfoveal choroidal thickness (r=0.2,
P=0.048).
CONCLUSION:
The current study demonstrated that the decreased serum VEGFR-2 level may be
considered in the development of AMD. Adropin does not seem to play a role in
the pathogenesis of AMD.
KEYWORDS: vascular endothelial
growth factor receptor-2; adropin; age-related macular
degeneration
DOI:10.18240/ijo.2016.04.13
Citation: Örnek N, Örnek K, Aydin
S, Yilmaz M, Ölmez Y. Serum
vascular endothelial growth factor receptor-2 and adropin levels in age-related
macular degeneration. Int J Ophthalmol 2016;9(4):556-560
INTRODUCTION
Age-related
macular degeneration (AMD) is a chronic progressive disease leading to visual
loss. Most patients with AMD have the dry type of the disease, however the dry
type AMD can lead to the wet type. Although only about 10% of people with AMD
develop the wet type, they make up the majority of those who have serious
vision loss from the disease. Several pathways have been implicated in the
pathogenesis of AMD. These are lipofuscin accumulation in retinal pigment
epithelium (RPE), choroidal ischaemia and oxidative damage. In dry type,
presence of yellow deposits (drusen) in the macula and progressive loss of the
RPE, choriocapillaris and photoreceptors occur. In wet type AMD, choroidal
neovascularization (CNV) breaks through to the neural retina, leaking fluid,
lipids and blood and leading to fibrous scarring.
Recently,
attention has been focused on vascular endothelial growth factor (VEGF) as a
therapeutic target. In wet type AMD, subretinal neovascularization (NV) may
originate from the retina or choroid[1].
The most common form of NV in AMD is CNV, in which vessels grow from the
choroid into the subretinal space or retina. VEGF-A is a major factor in the
development of CNV[2].
It has two main receptors, which are receptor tyrosine kinases, designated
vascular endothelial growth factor receptor-1 (VEGFR-1) and vascular
endothelial growth factor receptor-2 (VEGFR-2). VEGFR-2 is known as the major
angiogenic receptor for VEGF-A on endothelial cells[3]. In addition, protective functions
are mediated by VEGFR-2, as are autoregulatory functions of VEGF-A expression[4-5]. VEGFR-1 regulates VEGF activity
in the vascular endothelium by preventing binding of VEGF to VEGFR-2[6].
Among
the angiogenic factors, VEGF is the most important contributor to the
angiogenesis in AMD patients; however, there is limited evidence about the role
of anti-angiogenic molecules in AMD[7-8]. Recently, Uehara et al[9] found
decreased serum VEGFR-1 levels in wet type AMD compared to dry type and Sharma et
al[10]
reported an association between the serum levels of
VEGFR-2 and wet type AMD.
Adropin
was found in 2008 as a secreted protein involved in energy homeostasis,
metabolic adaptation to macronutrients and modulation of insulin sensitivity
and diabetes[11].
Lovren et al[12]
reported that adropin may also have non-metabolic properties including the
regulation of endothelial function. Adropin was expressed in endothelial cells
and improved angiogenesis-related responses. Authors concluded that adropin
potently upregulates VEGFR-2 in endothelial cells and that gene silencing of
VEGFR-2 significantly impaires the effects adropin had on modulating
endothelial cell survival and function[12].
To
date, VEGFR-2 and adropin in subtypes of AMD have not been investigated in one
study. In order to contribute in the clarification of the involvement of both
molecules in AMD, reviewing previous reports, in this study, we searched for a
relation between the serum levels of VEGFR-2 and adropin in AMD patients.
SUBJECTS AND METHODS
The
study included 98 (51 dry type, 47 wet type) AMD patients who were recruited
from the Ophthalmology Department of Kirikkale University during 2013.
Seventy-eight healthy age-matched volunteers were enrolled as control group.
Local Ethics Committee approved the study
protocol and informed consents were obtained from all participants. The study
was done in adherence to the tenets of the Declaration of Helsinki.
The
exclusion criteria were previous heart disease, renal/hepatic failure, acute
infection, hematologic disorder, presence of any chronic inflammatory and
autoimmune disease and any known malignancy. AMD group included patients with
dry or wet type AMD. Patients who received laser photocoagulation or
intravitreal drug injection during the last 6mo were not included into the
study.
Each
subject underwent a complete ophthalmological examination including
best-corrected visual acuity, measurement of intraocular pressure and slit lamp
examination. Fundus florescein angiography and optical coherence tomography
were performed to assess the posterior segment details. Height and weight measurements were used
to calculate the BMI. Peripheral blood samples were obtained from each
participant and serum levels of cholesterol, triglyceride (TRG), high density
lipoprotein (HDL), low density lipoprotein (LDL), glucose and HbA1c were
measured.
Serum
adropin levels were measured using an enzyme-linked immunosorbent assay (ELISA)
kit (Phoenix Pharmaceuticals, Burlingame, CA, USA) according to the
manufacturer’s instructions. The catalog number was 032-035. The detection range
of the kit was 0.01 to 100 ng/mL and the sensitivity was 0.5 ng/mL.
VEGFR-2 was also quantified using commercially available ELISA assays (eBioscience,
San Diego, CA, USA) according to manufacturer’s instruction. The lot number was
87412006 and the sensitivity was 7 pg/mL. Glucose was evaluated
in serum by the glucoseoxidase method. TRG, cholesterol, LDL, and HDL
concentrations were measured by an automated analyzer using commercially
available kits.
Statistical
Analysis
Statistical
analyses were carried out using the SPSS statistical software (SPSS for windows
10.0, Inc., Chicago, USA). One way analysis of variance (ANOVA) and Student’s t-test
were used for the analysis. Correlations were performed using Pearson’s
correlation coefficient. All data were expressed as mean ± standard deviation
(±SD). A P value less than 0.05
was considered statistically significant.
RESULTS
The
demographic characteristics and retinal thickness measurements of the study
groups are listed in Table 1. There was no statistically significant difference
between both groups in terms of age (P=0.6)
and sex (P=0.5). Compared to controls, AMD group had
significantly increased foveal retinal thickness, serum LDL and HDL levels and
significantly decreased subfoveal choroidal thickness (P=0.01, 0.047, 0.025 and
<0.001, respectively). Table 1 lists the clinical background and
biochemical characteristics of the two groups.
Table 1 Clinical
background and biochemical characteristics of the study groups
|
Parameters |
AMD group |
Dry type AMD group |
Wet type AMD group |
Controls |
1P |
|
Age
(a) |
73.43±9.18 |
74.14±8.57 |
72.66±9.83 |
72.52±8.53 |
0.6 |
|
Gender
(F/M) |
51/47 |
22/29 |
28/19 |
38/40 |
0.5 |
|
Weight
(kg) |
76.84±14.11 |
75.13±13.22 |
78.72±14.94 |
80.35±12.71 |
0.09 |
|
Height
(cm) |
159.29±11.0 |
160.45±10.33 |
158.02±11.74 |
159.87±9.20 |
0.7 |
|
BMI
(kg/m2) |
30.49±5.95 |
29.33±5.50 |
31.75±6.21 |
31.71±6.14 |
0.1 |
|
HbA1c
(%) |
6.29±1.01 |
6.10±0.85 |
6.50±1.13 |
6.04±0.73 |
0.06 |
|
Foveal
retinal thickness (µm) |
306.80±115.92 |
261.01±47.17 |
356.60±145.12 |
268.81±61.92 |
0.01 |
|
Subfoveal
choroidal thickness (μm) |
196.23±69.90 |
195.37±72.44 |
197.17±67.81 |
261.41±61.83 |
<0.001 |
|
VEGFR-2 (ng/mL) |
26.48±6.44 |
26.82±5.45 |
26.10±7.41 |
30.42±7.92 |
<0.001 |
|
Adropin
(ng/mL) |
6.17±3.19 |
5.57±2.60 |
6.81±3.65 |
5.79±2.71 |
0.4 |
|
Total
cholesterol (mg/dL) |
207.91±44.60 |
211.47±35.74 |
204.04±52.70 |
204.68±40.72 |
0.6 |
|
Triglyceride
(mg/dL) |
149.33±78.36 |
153.73±88.09 |
144.55±66.85 |
211.60±18.48 |
0.002 |
|
High-density
lipoprotein (mg/dL) |
52.03±15.51 |
54.88±18.89 |
48.94±10.04 |
47.40±10.42 |
0.025 |
|
Low-density
lipoprotein (mg/dL) |
127.57±37.95 |
128.96±32.14 |
126.06±43.70 |
117.19±30.76 |
0.047 |
|
Glucose
(mg/dL) |
118.95±48.85 |
115.65±52.36 |
122.53±45.03 |
108.29±27.93 |
0.08 |
AMD:
Age-related macular degeneration. 1AMD
group vs controls.
The
study demonstrated that AMD patients had significantly lower mean serum VEGFR-2
level compared to controls (26.48±6.44 vs 30.42±7.92 ng/mL, P<0.001). Both dry
and wet type AMD patients had significantly lower mean serum VEGFR-2 levels
(26.82±5.45 vs 30.42±7.92 ng/mL, P=0.05 and 26.10±7.41 vs
30.42±7.92 ng/mL,
P=0.003,
respectively) compared to control group. There was not any significant
difference in serum VEGFR-2 level between wet type and dry type AMD patients
(26.10±7.41 vs 26.82±5.45 ng/mL, P=0.5).
In
comparison to controls, mean serum adropin level was higher in AMD patients
(5.79±2.71 vs 6.17±3.19 ng/mL), but it did not show a significant
difference (P=0.4).
We observed a statistically insignificant decrease in dry type (5.57±2.60 vs 5.79±2.71 ng/mL, P=0.6) and a statistically insignificant increase in wet type AMD
patients (6.81±3.65 vs 5.79±2.71
ng/mL, P=0.07) compared to controls.
Comparison of wet type and dry type AMD patients did not reveal any significant
difference in serum adropin levels (6.81±3.65 vs 5.57±2.60 ng/mL, P=0.055). Figure
1 presents the serum levels of VEGFR-2 and adropin in AMD patients versus
controls.
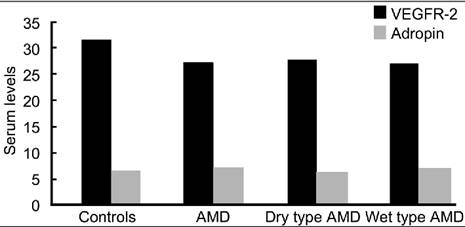
Figure 1 Serum levels of VEGFR-2
and adropin in AMD patients versus controls.
Serum
levels of VEGFR-2 in AMD patients had a significant negative correlation with
foveal retinal thickness (r =-0.226, P=0.025) and a
significant positive correlation with subfoveal choroidal thickness (r=0.2,
P=0.048).
Although the serum adropin concentration was higher in AMD patients than
controls (P=0.4),
there was no significant correlation between serum adropin level and retinal
and choroidal thickness (r=0.059, P=0.5 and r=-0.127,
P=0.2).
DISCUSSION
AMD is considered as the
main cause of visual loss in the elderly. In a large epidemiologic study, a
quarter of patients over the age of 75 were found to have some features of AMD,
about 80% of whom have dry type AMD and are at risk of converting to wet type
AMD. The onset of wet type AMD generally results in sudden vision loss and, if
left untreated, can cause permanent loss of sight. Wet type AMD accounts for
90% of the blindness attributable to AMD[13]. In wet type AMD, a major factor
in NV
which may exert from the retina or from the choroid is the VEGF. It is present
in a soluble and bound form and interacts with two receptors, VEGFR-1 and
VEGFR-2, to promote angiogenesis
and vascular integrity.
Endothelial
cells produce several factors that regulate cellular adhesion,
thromboresistance, smooth muscle proliferation and vessel wall inflammation.
Therefore, endothelial dysfunction is associated with several
pathophysiological conditions, including atherosclerosis, hypertension and
diabetes[14].
Recent studies supported the emerging concept of endothelial dysfunction in the
course of AMD. Clinical trials have found a link between endothelial
dysfunction and drusen formation or NV in AMD patients[15]. The protective role of adropin on the
endothelium has been shown previously[11,15-17]. In this study, we investigated
whether the serum levels of VEGFR-2 and adropin are related to the development
of AMD.
We
observed lower concentrations of serum VEGFR-2 in patients with AMD,
particularly in the wet type. Serum level of adropin was found to be less
important in the pathogenesis of AMD. VEGFR2 is the major positive signal
transducer for both physiological and pathological angiogenesis. The lowering
of circulating VEGFR-2 levels may be explained by a higher binding of the VEGF
to its R2 receptors to form VEGF-receptor complexes. Soluble isoforms of
VEGFR-1 and -2 are detected in blood circulation. These receptors are able to
bind their ligands, thereby controlling their biodisponibility and inhibiting
tumor or ischemia-induced angiogenesis[18-23]. Generally, circulating receptors
inactivate their ligands by binding with them, since the soluble receptor does
not possess the intracellular domain required to initiate signaling[24]. Both in vitro and in
vivo studies have shown that elevated levels of VEGFR-2 has anti-angiogenic
activity[25-26]. Therefore, a decrease of serum
VEGFR-2 level, the major proangiogenic signal transducer for VEGF, may be a
physiological response to promote angiogenesis in AMD eyes, particularly in the
wet type. We found a significant negative correlation between serum levels of
VEGFR-2 and foveal retinal thickness in AMD patients which is a measure of the
disease activation.
In
our study, serum VEGFR-2 levels in wet type AMD, though lower than in dry type,
did not produce a statistical significance. Possible reasons for the
insignificant difference for VEGR-2 levels could be in the ongoing, stepwise
process in AMD pathogenesis. It could be proposed that serum levels of VEGFR-2
in dry type AMD in our study may be related to earlier steps of the disease
process. Declining serum VEGFR-2 may be one of the the preliminary events
allowing VEGF to activate the proangiogenic endothelial cell state and to
induce permeability. The balance between angiogenic agent (like VEGF) and the
anti-angiogenic factors (like VEGFR-2),
which seems to be impaired in the AMD, and the degree to which the
anti-angiogenic agents are decreased might have determined the stage of macular
degeneration observed.
VEGFR-2
is activated by VEGF and is the initial pathway in modulating endothelial cell
survival and function. Lovren et al[12] showed the functions of adropin
including regulation of angiogenesis and increase in blood flow and capillary
density and reported its potential endothelial protective role. Wu et al[27] found lower serum adropin levels
in type 2 diabetes patients than in non-diabetic patients and reported that
adropin level was inversely associated with angiographic severity of coronary
atherosclerosis, suggesting that serum adropin may be a novel predictor of
coronary atherosclerosis. It is hard to conclude that higher serum adropin
levels in wet type AMD patients may be a response to pathological angiogenesis
in these patients or an initial sign of endothelial dysfunction and
atherosclerosis.
In
comparison to genetic and environmental factors, serological biomarkers are
less well known in AMD patients. Various immunological molecules and
inflammatory mediators have been identified at the site of AMD lesions. Serum
markers that have been associated with AMD development include elevated
superoxide dismutase, C-reactive protein, homocysteine and LDL etc[28-31]. Lip et al[7] found increased plasma VEGF levels
in AMD patients compared with age-matched controls. Interestingly, same Authors
did not find any significant difference between dry and wet AMD cases when
compared VEGF values. In a recent study, Tsai et al[8] found increased plasma VEGF levels
in AMD patients compared with controls. They found significantly higher plasma
VEGF levels in wet type AMD compared with dry type AMD. More recent studies
have reported elevated serum VEGFR-2 and reduced serum VEGFR-1 in patients with
neovascular AMD[9-10]. Serum level of adropin has not
been studied in AMD patients yet.
Another
issue is the relation of serum level of VEGFR-2 and adropin to the disease
severity. Sydorova and Lee[32]
measured high levels of VEGF in the serum and vitreous of proliferative
diabetic retinopathy and found serum VEGF levels were higher only in advanced
cases (proliferative vitreoretinopathy). As shown, there are still
controversies regarding the behaviour of the serological biomarkers in AMD. Surely,
an effective biomarker should be simple to analyze and clinically relevant.
Unfortunately, many of the candidate markers have yet to be standardized. Maybe
sooner, these biomarkers will be important tools for AMD researches by
providing a scale for evaluating disease progression.
In
summary, the decreased serum VEGFR-2 level seems to be related to the
development of AMD. Future researches should study whether decreasing serum
VEGFR-2 level promote the onset of wet type AMD and how VEGFR-2 levels decrease
in these patients. Although adropin does not seem to play a role in the
pathophysiology of AMD, it should be further studied at molecular levels in AMD
patients. As future studies continue to unravel the molecular biology of AMD,
both our understanding about this debilitating disease and the treatment
approaches will improve.
ACKNOWLEDGEMENTS
Conflicts of Interest:
Örnek
N, None; Örnek K, None; Aydin S, None; Yilmaz M, None;
Ölmez Y, None.
REFERENCES [Top]
1
Campochiaro PA. Ocular neovascularization. J Mol Med 2013;91(3):311-321. [CrossRef] [PubMed] [PMC free article]
2
Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular
endothelial growth factor in eye disease.Prog Retin Eye Res 2008;27(4):331-371.
[CrossRef] [PubMed] [PMC free article]
3
Shibuya M. Vascular Endothelial growth factor (vegf) and its receptor (VEGFR)
signaling in angiogenesis: a crucial target for anti- and pro-angiogenic
therapies. Genes Cancer 2011;2(12):1097-1105. [CrossRef] [PubMed] [PMC free article]
4
Byeon S, Lee SC, Choi SH, Lee HK, Lee JH, Chu YK, Kwon OW. Vascular endothelial
growth factor as an autocrine survival factor for retinal
pigment epithelial cells underoxidative
stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci
2010;51(2):1190-1197. [CrossRef]
[PubMed]
5
Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo
N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima
DT. Vascular endothelial growth factor-A is
a survival factor for retinal neurons and
a critical neuroprotectant during
the adaptive response to ischemic injury. Am J
Pathol 2007;171(1):53-67. [CrossRef]
6
Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Handb Exp
Pharmacol 2008;181:131-50. [CrossRef]
7
Lip PL, Blann AD, Hope-Ross M, Gibson JM, Lip GY.
Age-related macular degeneration is associated with increased vascular
endothelial growth factor, hemorheology and endothelial dysfunction.
Ophthalmology 2001;108(4):705-710.
[CrossRef]
8
Tsai DC, Charng MJ, Lee FL, Hsu WM, Chen SJ. Different
plasma levels of vascular endothelial growth factor and nitric oxide between
patients with choroidal and retinal neovascularization. Ophthalmologica
2006;220(4):246-251. [CrossRef]
[PubMed]
9
Uehara H, Mamalis C, McFadden M, Taggart M, Stagg
B, Passi S, Earle P, Chakravarthy U, Hogg RE, Ambati
BK. The reduction of serum soluble Flt-1 in patients with neovascular
age-related macular degeneration. Am J Ophthalmol 2015;159(1):92-100. [CrossRef] [PubMed] [PMC free article]
10
Sharma NK, Gupta A, Prabhakar S, Singh R, Sharma
S, Anand A. Single nucleotide polymorphism and serum levels of VEGFR2 are
associated with age related macular degeneration. Curr Neurovasc Res
2012;9(4):256-265. [CrossRef]
11
Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza
RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson
RA, Thearle M, Ferrante AW Jr, Mynatt RL,Burris TP, Dong
JZ, Halem HA, Culler MD, Heisler LK, Stephens
JM, Butler AA. Identification of adropin as a secreted factor linking
dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell
Metab 2008;8(6):468-481. [CrossRef]
[PubMed] [PMC free article]
12
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta
M, Al-Omran M, Teoh H, Verma S. Adropin is a novel regulator of
endothelial function. Circulation 2010;122(11):S185-192. [CrossRef] [PubMed]
13
Klein R, Klein BE, Knudtson MD, Meuer SM, Swift
M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular
degeneration: the Beaver Dam Eye Study. Ophthalmology 2007;114(2):253-262. [CrossRef] [PubMed]
14
Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction:
testing and clinical relevance. Circulation 2007;115(10):1285-1295. [PubMed]
15
Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai
N, Ridker PM.High-sensitivity C-reactive protein, other markers of
inflammation, and the incidence of macular degeneration in women. Arch
Ophthalmol 2007; 125(3): 300-305. [CrossRef] [PubMed] [PMC free article]
16
Celik A, Balin M, Kobat MA, Erdem K, Baydas A, Bulut M, Altas Y, Aydin S, Aydin
S. Deficiency of a new protein associated with cardiac syndrome X called
adropin. Cardiovasc Ther 2013;31(3):174-178. [CrossRef] [PubMed]
17
Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Molero-Ramirez
H, Tan HL, Bandla HP.Circulating adropin concentrations in pediatric
obstructive sleep apnea: potential relevance to endothelial function. J Pediatr
2013;163(4):1122-1126 [CrossRef]
[PubMed] [PMC free article]
18
Shibuya M. Differential roles of vascular endothelial growth factor receptor-1
and receptor-2 in angiogenesis. J Biochem Mol Biol 2006;39(5):469-478. [CrossRef]
19
Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch
U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A
stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and
ERK signaling. Circ Res 2008;103(9):916-918. [CrossRef] [PubMed] [PMC free article]
20
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor
signalling - in control of vascular function. Nat Rev Mol Cell Biol
2006;7(5):359-371. [CrossRef] [PubMed]
21
Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert
C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John
S, Neurath MF. VEGF receptor signaling links inflammation and
tumorigenesis in colitis-associated cancer. J Exp Med 2010;207(13):2855-2868. [CrossRef] [PubMed] [PMC free article]
22
Acobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ.
Adenoviral gene transfer with soluble vascular endothelial growth factor
receptors impairs angiogenesis and perfusion in a murine model of hindlimb
ischemia. Circulation 2004;110(16):2424-2429. [CrossRef] [PubMed]
23
Szentirmai O, Baker CH, Bullain SS, Lin N, Takahashi
M, Folkman J, Mulligan RC, Carter BS. Successful inhibition of
intracranial human glioblastoma multiforme xenograft growth via systemic
adenoviral delivery of soluble endostatin and soluble vascular endothelial
growth factor receptor-2: laboratory investiga:ion. J Neurosurg
2008;108(5):979-988. [CrossRef]
[PubMed] [PMC free article]
24 Igarashi
J, Michel T. S1P and eNOS regulation. Biochim Biophys Acta
2008;1781(9):489-495. [CrossRef]
[PubMed]
25
Kamba T, Tam BY, Hashizume H, Haskell A, Sennino
B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen
LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo
CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in
the normal adult microvasculature. Am J Physiol Heart Circ Physiol 2006;
290(2):H560-576. [CrossRef]
[PubMed]
26
Roeckl W, Hecht D, Sztajer H, Waltenberger J, Yayon
A, Weich HA. Differential binding characteristics and cellular inhibition
by soluble VEGF receptors 1 and 2. Exp Cell Res 1998;241(1):161-170. [CrossRef] [PubMed]
27
Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C, Fan
L. Low serum adropin is associated with coronary atherosclerosis in type 2
diabetic and non-diabetic patients. Clin Chem Lab Med 2014;52(5):751-758. [CrossRef] [PubMed]
28
Anand A, Sharma NK, Gupta A, Prabhakar S, Sharma
SK, Singh R. Superoxide dismutase1 levels in North Indian population with
age-related macular degeneration. Oxid Med Cell Longev 2013;2013:365046. [CrossRef] [PubMed] [PMC free article]
29
Seddon JM, Gensler G, Milton RC, Klein ML, Rifai
N.Association between C-reactive protein and age-related macular degeneration.
JAMA 2004;291(6):704-710. [CrossRef]
[PubMed]
30
Ghosh S, Saha M, Das D. A study on plasma homocysteine level in age-related
macular degeneration. Nepal J Ophthalmol 2013;5(2):195-200. [CrossRef]
31
Reynolds R, Rosner B, Seddon JM. Serum lipid biomarkers and hepatic lipase gene
associations with age-related macular degeneration. Ophthalmology
2010;117(10):1989-1995. [CrossRef] [PubMed] [PMC free article]
32
Sydorova M, Lee MS.Vascular endothelial growth factor levels in vitreous and
serum of patients with either proliferative diabetic retinopathy or
proliferative vitreoretinopathy. Ophthalmic Res 2005;37(4):188-190. [CrossRef] [PubMed]
[Top]