·Basic
Research··Current Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO·
Effect of long-term weightlessness on
retina and optic nerve in tail-suspension rats
Hong-wei
Zhao1,2, Jun Zhao2, Lian-Na Hu2, Jing-Nan
Liang3, Yuan-Yuan Shi2, Chuang Nie2, Chang-Yu
Qiu2, Xin-Shuai Nan4, Yu-Xin Li2,5, Fu-Lin Gao2,
Yi Liu2, Yu Dong6, Ling Luo2
1Chinese PLA General Hospital, Beijing 100039,
China
2Department of Ophthalmology, the 306th Hospital of PLA, Beijing 100101, China
3Institute of Microbiology, Chinese Academy of
Science, Beijing 100101, China
4Anhui Medical University, Hefei 230000, Anhui
Province, China
5Third Military Medical University, Chongqing
400038, China
6The First hospital of Jilin University, Changchun
130021, Jilin Province, China
Co-first authors: Hong-Wei Zhao and Jun Zhao
Correspondence to: Ling Luo. Department of Ophthalmology,
the 306th Hospital of PLA, Beijing 100101, China.
ling.luo@hotmail.com
Received:
2016-02-01
Accepted: 2016-04-29
Abstract
AIM: To evaluate the
effect of long-term weightlessness on retina and optic nerve in tail-suspension
(TS) rats.
METHODS: A stimulated
weightlessness model was established by suspending rats’ tail. After 12wk, the
ultrastructure and the number of optic nerve axons were observed by
transmission electron microscope. The number of survival retinal ganglion cells
(RGCs) was calculated by fluorescent gold retrograde labeling. Retina cells
apoptosis was detected by TUNEL staining. The function of optic nerve and
retina was evaluated by the visual evoked potential (VEP) and oscillatory
potentials (Ops).
RESULTS: The optic nerve
axons were swollen and sparsely aligned, and the lamellar separation and myelin
disintegration occurred after 12wk in TS rats. The density of optic nerve axons
was 32.23±3.92 (vs 37.43±4.13, P=0.0145), the RGCs density was 1645±46
cells/mm2 (vs 1867±54 cells/mm2 P=0.0000), the incidence rate of retinal cells apoptosis was
5.38%±0.53% (vs 4.75%±0.54%, P=0.0238), the amplitude of VEP-P100 was
15.43±2.14 µV (vs 17.67±2.17 µV, P=0.0424),
the latency of VEP-P100 was 69.05±5.34ms (vs
62.43±4.87ms P=0.0143) and the
sum amplitude of Ops was 81.05±8.34 µV (vs
91.67±10.21 µV, P=0.0280) in TS group
and the control group, respectively.
CONCLUSION: Long-term
weightlessness can induce the ultrastructural changes and functional depress of
the optic nerve, as well as retinal cell damages in TS rats.
KEYWORDS: weightlessness;
retina; optic nerve; tail suspension
DOI:10.18240/ijo.2016.06.06
Citation: Zhao HW, Zhao
J, Hu LN, Liang JN, Shi YY, Nie C, Qiu CY, Nan XS, Li YX, Gao FL, Liu Y, Dong
Y, Luo L. Effect of long-term weightlessness on retina and optic nerve in
tail-suspension rats. Int J Ophthalmol
2016; 6(6):825-830
INTRODUCTION
With
the depth of space exploration, the health and safety of space explorers
becomes more and more under
the spotlight and attracts much attention. The
effects on astronauts’ eyes during space flight were still not fully known. It
has been well-known that the significant ocular disorders could happen on
astronauts during space flight, including decreased visual acuity, hyperopic
shifts, papilledema, globe flattening, choroidal folds, retina damage and so on[1-5].
The collective effects of space radiation and weightlessness during space
flight are believed to be the main reasons [6-8]. However, whether
and how long-term weightlessness alone could contribute to the damage of optic
nerve still remains largely unknown.
Animal
studies have confirmed that weightlessness had remarkable effects on dorsal root
ganglion neurons, and the metabolism and excitation of neurons in the spinal
cord [9-12]. These studies provided convincing evidences that
weightlessness might cause central nervous system (CNS) damage. Since optic
nerve was composed of retinal ganglion cells (RGCs) axons without Schwann
cells, belongings to a part of the CNS, it was reasonable that weightlessness,
especially for long-term, might cause optic nerve damage. Therefore, we
hypothesized that long-term weightlessness may be associated with the injury of
optic nerve and further affect visual function. This study aimed to evaluate the
effect of long-term weightlessness on optic nerve and retina by using
tail-suspension (TS) rats as weightlessness models.
MATERIALS AND METHODS
Animals and Tail-suspension Model Adult
Sprague Dawley rats, without limitation of sex, weighing 200-300 g, aging 6-8wk, were
obtained from the Experimental Animal Center of Beijing Medical University.
Each rat was caged separately at 22℃-24℃and
controlled in light/dark cycles (12h/12h). One week later, 18 rats were
randomly assigned to two groups evenly: control group (without tail suspension)
and TS (with tail suspensions) group. TS model was made according to the method
described by Morey-Holton and Globus[13]. The rats were suspended by the
tail at an angle of about 30° from
the head down to avoid contact between the hind limbs and the ground. The rats
could walk freely on their forelimbs for access to food and water.
Experimental Design All rats received
visual evoked potential (VEP) and oscillatory potentials (Ops) tests at 12wk.
The ultrastructure and density of optic nerve axons was observed by
transmission electron microscope (TEM) in 3 rats of each group at 12wk. Three
rats in each group received retrograde fluorescent gold labeling with
intracranial injection at 11wk, and then was analyzed the density of survival
RGCs by counting RGCs labeled with fluorescent gold in retina flatmount under
fluorescence microscope at 12wk in each group. The incidence of RGCs apoptosis
was analyzed by TUNEL assay in other 3 rats of each group at 12wk.
Ultrastructure and number of optic nerve
axons Rats were
anesthetized, eyeballs and optic nerve were removed. Optic nerve (at a distance from retrobulbar 3-5 mm) was
taken and fixed in a cold solution consisting of glutaraldehyde and
paraformaldehyde, and postfixed with osmium tetroxide in the same buffer at 4℃ for
2h. Then the samples were dehydrated in a graded ethanol series, and treated
with propylene oxide, and finally embedded in Spurr’s resin. Ultrathin cross
sections of optic nerve (approximately 50 nm in thickness) were made by an
ultramicrotome (Leica UC7, Germany) with a diamond knife. The stained cross
sections were examined with a transmission electron microscope (JEM-1400, JEOL
Ltd., Tokyo, Japan) operated at an accelerating voltage of 80 kV. Three
sections of each sample were randomly selected. The number of optic nerve axons
was manually counted in 5 fields randomly selected on each section and
averaged.
Retrograde fluorescent gold
labeling It
was performed according to the literature[14]. Briefly, rats were deeply
anesthetized (10% hydrate of chlorine aldehyde), then placed in a small
stereotactic instrument. The skull was opened. The Bregma point was identified
and the injection points overlying the lateral geniculate body was marked. The
holes were drilled at demarcated points with a 25 Gauge needle. 2 µL of 2% fluorescent gold (Biotium, 80014, USA) was
injected into the superior colliculus and lateral geniculate body of each
hemisphere through the bony surface of the brain by using a Hamilton syringe.
And then the incision of skin was sutured.
Retina flatmount and retinal
ganglion cellscounting Rats
were anesthetized, eyeballs were enucleated and postfixed for 1h in 4%
paraformaldehyde. Cornea and the lens were removed and the eyecups were
incubated in phosphate buffer saline (PBS). The whole retina was then carefully
dissected, flat mounted on slides, and cover slipped. Fluorescent RGCs were
observed with fluorescent microscope (Japan OlympusBX-53, Fluorescence
attachment). Each retina was divided into four quadrants (superior, inferior,
nasal and temporal), and images of retina within 2.0 mm from the center of the
optic disc in each quadrant were obtained. The size of counted area in each
quadrant was 0.153 mm2 (450×340 µm2).
Fluorescent RGCs on the images were measured using Image Pro plus 6.0. Finally,
the number of RGCs was obtained by dividing the area and expressed as number
per square millimeter.
TUNEL assay and the analysis of retinal
ganglion cells apoptosis TUNEL
assay was used for RGCs apoptosis detection. It was performed according to the
CFTM488A TUNEL Assay Apoptosis Detection Kit (American Biotium Corporation,
30063) protocol. Negative controls were incubated with TUNEL reaction buffer
without TdT Enzyme. Photographs were taken by using a fluorescent microscope
(OlympusBX-53, Fluorescence attachment, Japan). Nine sections were randomly
selected in each eye, and one field was randomly selected on each section.
TUNEL-positive cell counts were calculated manually as percentages of total
cell number.
Visual evoked potential and oscillatory
potentials waves cording After
dark-adaptation, rats were anesthetized with intraperitoneal 10% chloral
hydrate. VEP signals were recorded in the scalp covering the visual cortex
using a stainless steel recording electrode (Gift from The 4th
Military Medical University) placed subcutaneously 1 cm anterior to the
midpoint of a line connecting the two back ear edges. A stainless steel
reference electrode was placed in the mouth, and a stainless steel grounding
electrode was placed in the tail. The flash stimulus parameter was 3.5 cd·s·m-2,
1.3 Hz. The amplitude and latency of the VEP-P100 component were recorded
automatically (GUOTE MEDICAL, V8.1, China). After VEP cording, rats were
dilated pupils using 1% tropicamide eye drops. The corneal surfaces were
anesthetized using 0.4% hydrochloric acid oxybuprocaine eye drops. The
reference electrode was placed on the foreheads of the rats, the ground
electrode was placed on their ears, and the ring-recording electrode was placed
on the surface of the corneas of the rats. The pass band of full-field
stroboscopic white light stimulus was 75-300 Hz. The flash stimulus parameter
was 3.0 cd·s·m-2.
The sum of Ops amplitudes was recorded automatically.
Statistical Analysis The experimental
data are expressed as the mean±standard deviation and was analyzed by Student’s
t-test. Each P-value was calculated by two-tail student’s t-test with P<0.05
considered significant.
RESULTS
Long-term Weightlessness Caused Optic
Nerve Injury and Reduced the Density of Optic Nerve Axons in Tail-suspension
Rats To
observe the effect of long-term weightlessness on optic nerve axons in TS model
rats, the ultrastructure of cross section of the optic nerve was observed by
TEM at 12wk after TS. Normal optic nerve axons are closely aligned and have
compact myelin, normal microfilaments, microtubules and mitochondria (Figure
1A). While in TS group, optic nerve axons were swollen and sparsely aligned,
and were observed to have abnormal proliferation of neural connective tissue
between axons, lamellar separation and disintegration of myelin (Figure 1B).
The number of optic nerve axons was 32.23±3.92 in TS group, and 37.43±4.13 in
control group respectively (P=0.0145).
Optic nerve axons density was decreased approximately 15% in the TS group
compared with that of the control group (Figure 1C).

Figure
1 The
ultrastructure of optic nerve axons by TEM and comparison of optic nerve axon
density in rats between control group and TS group A: Optic nerve
axons were closely aligned, and had compact myelin in normal rats (arrow); B:
Optic nerve axons were swelling and sparsely aligned, even occurred in the
lamellar separation and disintegration of myelin (arrow); C: The number of
optic nerveaxons in TS group was 32.23±3.92 in TS group, vs 37.43±4.13 in control group in the same magnification field of
view (P=0.0145). Scale bar=0.5 µm.
Long-term Weightlessness Decreased the
Number of Survival Retinal Ganglion Cells in Tail-suspension Rats To explore the
effect of long-term weightlessness on RGCs number. We countered the number of
survival RGCs in retina flatmount at 12wk after TS. RGCs were presented as
green-fluorescent spots. The number of RGCs was 1645±46 cells/mm2 in
TS rats group, and 1867±54 cells/mm2 in control group (Figure 2A, 2B,
P=0.0000). RGCs density was decreased
13% in the TS group compared with that of the control group (Figure 2C).

Figure
2 RGCs numbers in
rats with fluorescent gold retrograde-labeling between control group and TS
group A:
Images of representative retinal flat mounts in control group. A lot of RGCs
were present as big and bright green-fluorescent spots in retinal preparations
(×400 magnification). B: Images of representative retinal flat mounts in TS
group. The number of big and bright green-fluorescent spots of RGCs in retinal
preparations was decreased (×400 magnification). C:The RGCs density
was 1645±46 cells/mm2 in TS group, vs 1867±54 cells/mm2 in
control group (P=0.0000).
Long-term Weightlessness Increased the
Incidence Rate of Retinal Cells Apoptosis in Tail-suspension Rats In order to
examine the effect of long-term weightlessness on retinal cells apoptosis in TS
model rats. Next, we detected retinal cells apoptosis using the TUNEL assay at
12wk after TS. The TUNEL results showed there are very few numbers of retinal
apoptosis cells presented as bigger green-fluorescent spots in normal rats (Figure
3A), while the number of retinal apoptosis cells, especially in RGCs layer, was
significantly increased in TS rats group (Figure 3B). The incidence rate of
retinal cells apoptosis was 4.75%±0.54% in control
group, and was 5.38%±0.53% in TS group (P=0.0238).
The incidence rate of retinal cells apoptosis was increased 13% in the TS group
compared with that of the control group (Figure 3C).

Figure
3
Retinal cells apoptosis in rats by TUNEL
assay between control group and TS group A: Images of representative
retinal cells apoptosis in control group. Apoptotic retinal cells were present
as big and bright green fluorescent spots (arrow, ×400 magnification). B:
Images of representative retinal cells apoptosis in TS group. The number of
apoptotic retinal cells presented as green fluorescent spots was increased,
especially in RGCs layer (arrow, ×400 magnification). C: The incidence rate of
retinal cells apoptosis was 4.75±0.54% in control group, vs 5.38±0.53% in TS group (P=0.0238).
Long-term Weightlessness Depressed
Visual Evoked Potential and Oscillatory Potentials Waves in Tail-suspension
Model Rats In order to value the function of optic nerve and
retina after long-term weightlessness in TS model rats. We detected VEP and OPs
waves of rats by visual electrophysiology instrument at 12wk after TS. The
results showed the amplitude of VEP-P100 was decreased approximately 13% in the
TS group (15.43±2.14 µV) compared with that of the control group (17.67±2.17
µV). There was statistical significance of amplitude of VEP-P100 between two
groups (P=0.0424) (Figure 4A). The
latency of VEP-P100 was increased approximately 11% in the TS group
(69.05±5.34ms) compared with that of the control group (62.43±4.87ms). There
was statistical significance of latency of VEP-P100 between two groups (P=0.0143) (Figure 4B). The sum amplitude
of Ops was decreased approximately 13% in the TS group (81.05±8.34 µV) compared
with that of the control group (91.67±10.21 µV). There was statistical significance
of the sum amplitude of Ops between two groups (P=0.0280) (Figure 4C).
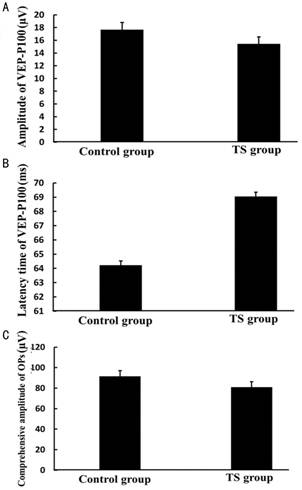
Figure 4 The amplitude and latency of
VEP-P100 and the sum amplitude of Ops in control group and TS group A:
The amplitude of VEP-P100 was 17.67±2.17 µV in control group, vs 15.43±2.14 µV in TS group (P=0.0424); B: The latency of VEP-P100
was 62.43±4.87ms in control group, vs
69.05±5.34ms in TS group (P=0.0143);
C: The sum amplitude of Ops was 91.67±10.21 in control group, vs 81.05±8.34 in TS group (P=0.0280).
DISCUSSION
In
this study, we demonstrated that the optic nerve ultrastructure was damaged, including
optic nerve axons swelling,sparsely
alignment, lamellar separation and even disintegration of myelin. The density
of optic nerve axons and the numbers of survival RGCs was significantly
decreased. The incidence of RGCs apoptosis was increased. VEP and Ops waves
were exacerbated due to weightlessness in TS rats.
Weightlessness
can cause the disorders of multiple organs or organizations, such as bone,
muscle, cardiopulmonary, immune system and some ocular disorders as well[15-18].
Mader et al[2] reported that
5 out of 7 astronauts in space for 6mo happened optic papilla edema, suggesting
that optic nerve could be damaged during space flight. However, regardless
space radiation factor, whether and how the weightlessness factor alone could
induce this damage still remains unclear. Our study demonstrated that weightlessness in TS
rats can cause both optic nerve injury and RGCs apoptosis. To our knowledge, it
was the first report on documenting it.
Although
the mechanism about optic nerve injury by weightlessness factor is not
completely understood, we speculate that it may be related to the following
factors: 1) increased intracranial pressure. Loss of gravitationally induced
cranial outflow of blood in the vertebral veins and collaterals, may lead to intracranial venous
hypertension and subsequently cause papillary edema. Long-term papillary edema
could produce expansion of optic nerve sheath, which further compress the optic
nerve; 2) intraocular pressure (IOP). Long-term high IOP in a state of weightlessness
could compress the optic disc and result in optic nerve injury; 3) imbalanced
translaminar pressure difference (TLPD). The difference in IOP
and cerebrospinal fluid (CSF) across
the lamina cribrosa is known as the TLPD. It was reported astronauts returning
from prolonged space flight on the International Space Station with papilledema
[2].
But papilledema has not been observed in shorter duration space flight. A study
has demonstrated that CSF no longer pools in the caudal spinal column as it
does in the upright position on earth. Instead, CSF diffuses throughout the
subarachnoid space resulting in a moderate but persistently elevated cranial
CSF pressure,
including the region just posterior to the lamina cribrosa known as the optic
nerve subarachnoid space. This small but chronically elevated CSF could lead to papilledema when CSF pressure is greater
than the IOP[19].
Apart
from optic nerve injury, our study further demonstrated that the numbers of
survival RGCs was significantly decreased and might subsequently contributed to
a decline of visual function which evaluated by VEP. A recent report revealed
that spaceflight conditions induce oxidative damage that results in significant
apoptosis of retina cells in rats, especially inner nuclear layer and ganglion
cell layer[8]. But the conclusion of above study cannot be excluded
the result of joint action both weightlessness and space radiation. Our study
indicated that long-term weightlessness alone could enough increase the
apoptosis RGCs, as well as that of retinal cells.
Retinal
ischemia might play an important role in the process of RGCs and retinal cells’
apoptosis during weightlessness. A decreased Ops in electroretinogram (ERG) was
found in this study is as an evidence for this speculation. Ops is the
sub-component of ERG, it can objectively and sensitively reflect inner retinal
blood circulation. Previous studies have confirmed that the weightlessness
could cause ocular hemodynamics chang[2,4] and vascular endothelial cell
apoptosis in rats of spaceflight conditions[8]. Redistribution of blood in the
head and face due to weightlessness can cause ocular venous congestion.
Short-term redistribution of blood produces autonomous adaptation, but which
for long time will produce a series pathophysiology retinal changes. Our study
provided a proof that the function of the retina was damaged might through
changing inner retinal blood circulation due to long-term weightlessness in TS
rats.
Notably,
other important factors could not be excluded in the process of apoptosis of
RGCs and retinal cells, such as oxidative stress. Several studies have
suggested that weightlessness resulted in increased oxidative stress in the CNS
[20-21],
which might have profound implications in the pathogenesis of retinal cells death
[22-23].
Moreover, it was reported that Bcl-2 signaling pathways may represent an event
upstream of the retina cells apoptosis step in rats of spaceflight conditions [8].
However, more investigations should be taken to clarify this issue.
In
summary, our study confirmed that long-term weightlessness alone could enough
cause morphological and functional optic nerve damage, and induce RGCs and
retinal apoptosis. But a simulated weightlessness animal model will never
reflect fully the picture of actual spaceflight in human. This limitation was
well known and needed to further overcome to extrapolate reasonably from a
simulated weightlessness animal model to human. Nevertheless, these simulated
animal studies do provide ideas to further evaluate the optic nerve and retina
changes in human weightlessness.
ACKNOWLEDGEMENTS
Foundation: Supported by
the National Natural Scientific Foundation of China (No. 81271016).
Conflicts of Interest: Zhao HW, None; Zhao J, None; Hu
LN, None; Liang JN, None; Shi
YY, None; Nie C, None; Qiu
CY, None; Nan XS, None; Li YX, None; Gao
FL, None; Liu Y, None; Dong
Y,
None; Luo L, None.
REFERENCES
1 Mader TH, Gibson CR, Caputo M, Hunter N, Taylor G, Charles J,
Meehan RT. Intraocular pressure and retinal vascular changes during transient
exposure to microgravity. Am J Ophthalmol
1993;115(3):347-350. [CrossRef]
2 Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe
flattening, choroidal folds, and hyperopic shifts observed in astronauts after
long-duration space flight. Ophthalmology
2011;118(10):2058-2069. [CrossRef] [PubMed]
3 Peters BT, Miller CA, Brady RA,
Richards JT, Mulavara AP, Bloomberg JJ. Dynamic visual acuity during walking after
long-duration spaceflight. Aviat, Space,
Environ Med 2011;82(4):463-466. [CrossRef]
4 Shinojima A, Iwasaki K, Aoki K, Ogawa
Y, Yanagida R, Yuzawa M. Subfoveal choroidal thickness and foveal retinal
thickness during head-down tilt. Aviat,
Space Environ Med 2012;83(4):388-393. [CrossRef] [PubMed]
5 Wiener TC. Space obstructive syndrome:
intracranial hypertension, intraocular pressure, and papilledema in space. Aviat Space Environ Med
2012;83(1):64-66. [CrossRef]
6 Tombran-Tink J, Barnstable CJ. Space
flight environment induces degeneration in the retina of rat neonates. Adv Exp Med Biol 2006;572:417-424. [CrossRef] [PubMed]
7 Tombran-Tink J, Barnstable CJ. Space
shuttle flight environment induces degeneration in the retina of rat neonates. Gravit Space Biol Bull 2005;18(2):97-98.
[PubMed]
8 Mao XW, Pecaut MJ, Stodieck LS,
Ferguson VL, Bateman TA, Bouxsein M, Jones TA, Moldovan M, Cunningham CE, Chieu
J, Gridley DS. Spaceflight environment induces mitochondrial oxidative damage
in ocular tissue. Radiat Res
2013;180(4):340-350. [CrossRef]
[PubMed]
9 Gorbunova AV. Effects of space-flight
factors on cytochemical characteristics of the motor analyzer neurons. Vestn Ross Akad Med Nauk 2010;(5):15-21.
[PubMed]
10 Krasnov IB, D'iachkova LN. The
acceleration of ultrastructure changes of synapses in somatosensory cortex of
the rats at repeated simulation of weightlessness effects. Aviakosm Ekolog Med 2006;40(2):53-54. [PubMed]
11 Ren JC, Fan XL, Song XA, Zhao XH,
Chen MX, Shi L. Prolonged hindlimb unloading leads to changes in
electrophysiological properties of L5 dorsal root ganglion neurons in rats
after 14 days. Muscle Nerve 2012;45(1):65-69.
[CrossRef] [PubMed]
12 Yang W, Fan XL, Zhang H, Di Wu S,
Song XA. Effects of hindlimb unloading and reloading on c-fos expression of
spinal cord evoked by vibration of rat Achille tendon. Neurosci Lett 2008;439(1):1-6. [CrossRef] [PubMed]
13 Morey-Holton ER, Globus RK. Hindlimb
unloading rodent model: technical aspects. J
Appl Physiol 2002;92(4):1367-1377. [CrossRef]
[PubMed]
14 Chiu K, Lau WM,
Yeung SC, Chang RC, So KF. Retrograde labeling of retinal ganglion cells by
application of fluoro-gold on the surface of superior colliculus. J Vis Exp 2008;(16).pii:819.
15 Arfat Y, Xiao WZ, Iftikhar S, Zhao F,
Li DJ, Sun YL, Zhang G, Shang P, Qian AR. Physiological effects of microgravity
on bone cells. Calcif Tissue Int
2014;94(6):569-579. [CrossRef]
[PubMed]
16 Stein TP. Weight, muscle and bone
loss during space flight: another perspective. Eur J Appl Physiol 2013;113(9):2171-2181. [CrossRef] [PubMed]
17 Campbell MR, Charles JB. Historical
Review of Lower Body Negative Pressure Research in Space Medicine. Aerosp Med Hum Perform
2015;86(7):633-640. [CrossRef]
[PubMed]
18 Hoff P, Belavy DL, Huscher D, Lang A,
Hahne M, Kuhlmey AK, Maschmeyer P, Armbrecht G, Fitzner R, Perschel FH, Gaber
T, Burmester GR, Straub RH, Felsenberg D, Buttgereit F. Effects of 60-day bed
rest with and without exercise on cellular and humoral immunological
parameters. Cell Mol Immunol 2015;12(4):483-492.
[CrossRef] [PubMed] [PMC free
article]
19 Berdahl JP, Yu DY, Morgan WH. The
translaminar pressure gradient in sustained zero gravity, idiopathic
intracranial hypertension, and glaucoma. Med
Hypotheses 2012;79(6):719-724. [CrossRef] [PubMed]
20 Chen HL, Qu LN, Li QD, Bi L, Huang
ZM, Li YH. Simulated microgravity-induced oxidative stress in different areas
of rat brain. Sheng Li Xue Bao
2009;61(2):108-114. [CrossRef]
[PubMed]
21 Zhang R, Ran HH, Ma J, Bai YG, Lin
LJ. NAD(P)H oxidase inhibiting with apocynin improved vascular reactivity in
tail-suspended hindlimb unweighting rat. J
Physiol Biochem 2012;68(1):99-105. [CrossRef]
22 Roy S, Trudeau K, Roy S, Tien T,
Barrette KF. Mitochondrial dysfunction and endoplasmic reticulum stress in
diabetic retinopathy: mechanistic insights into high glucose-induced retinal
cell death. Curr Clin Pharmacol 2013;8(4):278-284.
[CrossRef] [PubMed]
23 Li H,
Wang B, Zhu C, Feng Y, Wang S, Shahzad M, Hu C, Mo M, Du F, Yu X. 17β-estradiol impedes
Bax-involved mitochondrial apoptosis of retinal nerve cells induced by
oxidative damage via the phosphatidylinositol 3-kinase/Akt signal pathway. J Mol Nneurosci 2013;50(3):482-493.
[Top]