INTRODUCTION
Diabetic retinopathy (DR) is one of the microvascular complications of diabetes, and represents the leading cause of decreased vision in working people. DR is mostly a vascular disease and the development of macular edema and proliferative retinopathy are major causes of visual impairment[1]. Over the last years a lot of work has been done on early diagnosis of DR and on looking for new ocular diagnostic tools useful in evaluating patients affected by diabetes[2].
The retina contains two main capillary plexuses: superficial capillary plexus lies in the nerve fiber layer or ganglion cell layer, while deep capillary plexus is located within the inner nuclear layer[2]. The foveola and the immediate parafoveal retina is lack of capillaries, making this area dependent on the blood supply from the choriocapillaris. This area represents the foveal avascular zone (FAZ) and pathologic conditions that feature retinal capillary dropout, such as diabetes, lead to an enlargement and irregular margins of the FAZ.
Fluorescein angiography (FA) is an essential diagnostic tool in DR staging[3]. Indeed, FA can identify both primary vascular lesions (e.g. microaneurysms and intraretinal microvascular abnormalities), and retinal non-perfused areas and presence of neovascularizations. However, FA is an exam in which complications can happen, as nausea and, infrequently,anaphylaxis[4]. Moreover, FA is a two-dimension imaging exam and the latter feature does not let us to separately visualize superficial and deep capillaries[2].
Introduction of optical coherence tomography angiography(OCTA) has allowed the opportunity both to study retinal vasculature without the need for dye injection and to image the two plexuses. Moreover, OCTA is able to image and evaluate both the presence of retinal vascular abnormalities and neovascularizations associated to diabetes[5].
In the current study, using OCTA, we investigated the FAZ area and retinal vascular densities in patients affected by type 2 diabetes compared to healthy controls. The main purpose of the present study was to assess FAZ area and the parafoveal vessel density both in the superficial and deep plexus throughout the different stages of DR and to assess reproducibility of both FAZ area and parafoveal vessel density (PRVD) measurements.
SUBJECTS AND METHODS
Subjects Sixty diabetic patients with type 2 diabetes mellitus were enrolled in the study. Patients consecutively presented at the Department of Ophthalmology, University G. d’Annunzio of Chieti-Pescara, between June 2014 and March 2015. Twenty age-matched healthy subjects were included as controls. The Institutional Review Board approved this study and patients signed informed consent to the use of their data. The study adhered to the tenets of the Declaration of Helsinki.
Criteria for inclusion were: 1) age >18 years old; 2) bestcorrected visual acuity (BCVA) greater than 0.5 logMAR in the study eye at baseline examination (to ensure proper execution of examination); 3) confirmed diagnosis of diabetes.The exclusion criteria were: 1) any ocular surgery (included intravitreal injections) in the study eye in the last 6mo; 2) laser treatment in the study eye; 3) history of glaucoma; 4) media opacity in the study eye.
Study Protocol All recruited patients underwent a complete ophthalmic evaluation, including assessment of BCVA,tonometry, slit-lamp biomicroscopy, and indirect fundus ophthalmoscopy. Furthermore, all patients were tested by means of XR Avanti® Angio Vue OCTA (Optovue Inc., Fremont,CA, USA) and FA with Heidelberg Retina Angiograph 2 (HRA2)(HRA+OCT Spectralis: Heidelberg Engineering, Heidelberg,Germany).
DR staging were defined according to the Clinical Diabetic Retinopathy Scale proposed by the Diabetic Retinopathy Project Group[6]. The stages are as follows: I) no DR; II) mild non proliferative diabetic retinopathy (NPDR); III) moderate NPDR; IV) severe NPDR; V) proliferative diabetic retinopathy(PDR).
Outcome measures included: 1) FAZ are ameasured by both OCTA and FA; 2) parafoveal superficial vessel density(PSVD); 3) parafoveal deep vessel density (PDVD).
Procedures Spectral domain-optical coherence tomography angiography with XR Avanti XR Avanti® AngioVue OCTA (Optovue Inc.,Fremont, CA, USA) is a device with a high-speed of 70 000 axial scans per second, using a light source of 840 nm, and an axial resolution of 5 μm. The AngioVue OCTA system based on SSADA algorithm (Version: 2015.100.0.13) uses blood flow as intrinsic contrast. Indeed, the flow is detected as a variation over time in the speckle pattern formed by interference of light scattered from red blood cell (RBC) and adjacent tissue structure[7-8].
Before imaging, each subject’s pupils were dilated with a combination of 0.5% tropicamide and 10% phenylephrine.Study participants underwent spectral domain-optical coherence tomography (SD-OCT) imaging following a protocol that included AngioVue OCT 3D volume set of 3 mm×3 mm,consisting of 304×304 pixels in the transverse dimension. An internal fixation light was used to center the scanning area.
One FastX (horizontal raster) set and one FastY (vertical raster) set were performed for each acquisition scan. Scans with low quality (i.e. if the subject blinked or if there were significant motion artifacts) were excluded and repeated until good quality was achieved. Three scans for each patient were captured (all with a signal straight index >60).
Vascular layer segmentation Vascular retinal layers were visualized and segmented as previously described[9]. To evaluate the superficial retinal plexus we used a layer thickness of 60 micron from the inner limiting membrane, in order to include all the vessels of this plexus. Therefore, to visualize the deep retinal plexus we used a 30 micron thick layer from the inner plexiform layer, for the purpose of visualizing the plexus in its entirely. Then in order to remove projection artifacts from the inner vascular plexus the slab section was moved slight posteriorly centered on the outer plexifom layer. Two observers, independently, checked and set the segmentation, as better as they judged.
Vessel density analysis Objective quantification of vessel density was evaluated for each scan using the SSADA software. Quantitative analysis was performed on the OCTA en-face images using the AngioVue software. PRVD was defined as the percentage of area occupied by vessels in a ringshaped region of interest (ROI) centered on the center of the FAZ with an inner radius of 1.00 mm and an outer radius of 2.5 mm. AngioVue software automatically outputs the flow area value within the ROI.
The vessel density is calculated using the formula previously described[7-8], as follows:![]() , where V is 1 when the OCTA value is above a background threshold and 0 otherwise. A is the area of interest.
, where V is 1 when the OCTA value is above a background threshold and 0 otherwise. A is the area of interest.
Because as we captured three scans for each patient and as two observers evaluated each scan, we obtained six values for eachmeasure. For the comparison among the groups we considered the mean value between the six obtained measures.
Table 1 FAZ and vessel density in diabetic patients and controls, evaluated by means of OCTA and FA
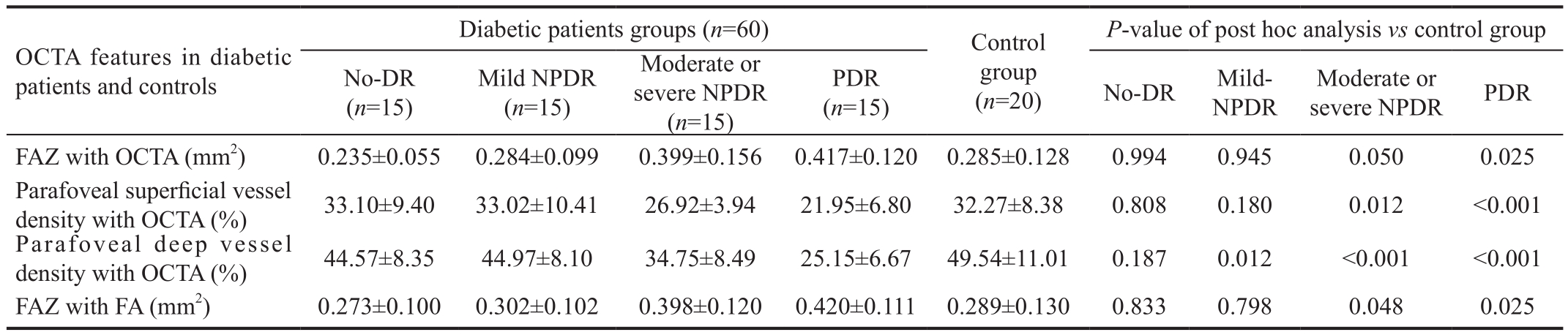
FAZ: Foveal avascular zone; OCTA: Optical coherence tomography angiography; FA: Fluorescein angiography; No-DR group: Diabetes patients without any sign of diabetic retinopathy; Mild-NPDR group: Diabetes patients with signs of mild non proliferative diabetic retinopathy;Moderate or severe NPDR group: Diabetes patients with signs of moderate or severe NPDR; PDR group: Diabetes patients affected by proliferative diabetic retinopathy.
Foveal avascular zone analysis FAZ area was measured in square millimeters (mm2): brie fl y, a non- flow measurement on a superficial reference plane (superficial vascular plexus) of en-face projection was selected. The user manually fine-tuned the plane in order to maximize the visualization of the retinal capillary bed. Upon clicking on the center of the FAZ the software automatically calculated the area. Also in this case,two observers evaluated the three patient scans, obtaining six FAZ measures for each patient.
Retinal fluorescein angiography Retinal FA was performed after pupil dilatation with a combination of 1% tropicamide and 10% phenylephrine. Consecutive digital fundus images were acquired by the Heidelberg Spectralis instrument in highresolution mode. Highest quality images were chosen by a retina specialist for analysis.
Foveal avascular zone area measurement Two blinded observers calculated FAZ area using the following steps:adjusting image contrast to enhance the FAZ; encircling the FAZ borders and measuring the area within this border. FAZ area was evaluated on FA image.
Statistical Analysis This study was designed to estimate reproducibility of FAZ area measurement. Assuming a withinsubject standard deviation of 13% and three measurements by means of HRA2 and OCTA per subject from two observers,using a Bland formula the sample size required to estimate the width of the 95% con fidence interval (CI) within 13% was 60 subjects.The quantitative variables were summarized as mean and standard deviation (SD), qualitative variables as frequency and percentage. A Shapiro-Wilk’s test was performed to evaluate the departures from normality distribution for each variable.
A plot of the difference of the measurement (FAZ area and vessel density) between observers for each subject against their mean was generated to detect any possible relationship between the measurement error and the true value. Bland and Altman method was applied to evaluate the limits of agreement.Lin’s concordance correlation coefficient (CCC) was calculated along with the 95% CI to assess the interobserver reproducibility of measurements. At each subject was attributed the mean value between observer 1 and observer 2 for FAZ area and PRVD. The resulted variables were analysed using different analysis of variance (ANOVA) test. In all models post hoc analysis, were used to compare means of different parameters (FAZ area and vessel density) between groups. Statistical analysis was performed using the computing environment R (R Development Core Team, 2005).
RESULTS
A total of 60 eyes of 60 diabetic patients (35 males and 25 females; mean age 65.6±5.7y, range 54-72y) were enrolled for the study. Diabetic patients were graded according to the Clinical Diabetic Retinopathy Scale[6]. There were 15 eyes without any sign of DR, 15 eyes with mild NPDR), 15 eyes with moderate or severe NPDR, 15 eyes with PDR. A control group of 20 healthy age-matched subjects (12 males and 8 females; mean age 64.5±7.6y, range 54-73y; P=not significant)was selected for statistical comparisons.
BCVA was 0.00±0.01 logMAR in healthy controls, 0.00±0.02 logMAR in patients without DR, 0.04±0.03 logMAR in patients with mild NPDR, 0.18±0.09 logMAR in subjects affected by moderate or severe NPDR and 0.34±0.15 logMAR in PDR patients (P<0.01, ANOVA test).
Foveal Avascular Zone Evaluation Mean FAZ area with OCTA was 0.285±0.128 mm2 in the control group and 0.235±0.055 mm2 in patients affected by diabetes and without DR. Considering patients affected by DR, FAZ area was 0.284±0.099 mm2, 0.399±0.156 mm2 and 0.417±0.120 mm2 in mild NPDR group, moderate and severe NPDR group and PDR group, respectively (P<0.001, ANOVA test; Table 1).
After comparing patient groups with control group, FAZ area was statistically significant increased in moderate or severe NPDR group (P=0.050, post hoc analysis) and PDR group(P=0.025, post hoc analysis; Table 1).
Mean FAZ area using FA image was 0.289±0.130 mm2 in the control group and 0.273±0.100 mm2 in patients affected by diabetes and without DR. Considering patients affected by DR, FAZ area was 0.302±0.102 mm2, 0.398±0.120 mm2 and 0.420±0.111 mm2 in mild NPDR group, moderate and severe NPDR group and PDR group respectively (P=0.050, ANOVA test).
After comparing patient groups with control group, FAZ area was statistically significant increased in moderate or severe NPDR group (P=0.048, post hoc analysis) and PDR group(P=0.025, post hoc analysis; Table 1).
Because of FAZ area measuring was done by two different investigators, we were able to compare FA and OCTA interobserver variability in FAZ measurement (Figure 1). OCTA showed a less inter-observer variability than FA in measuring FAZ area: CCC was 0.829 (95%CI: 0.736-0.891) P<0.001 for FA and 1.000 (95%CI: 0.999-1.000) P<0.001 for OCTA indicating very good agreement between the investigators for the two tested measurements.
Vessel Density Evaluation by Means of Optical Coherence Tomography Angiography PSVD was 32.27%±8.38% in the control group and 33.10%±9.40% in patients affected by diabetes without DR. Considering patients affected by DR,PSVD was 33.02%±10.41% in patients with mild NPDR,26.92%±3.94% in patients with moderate or severe NPDR and 21.95%±6.80% in PDR patients (P=0.006, ANOVA test;Figure 2; Table 1).
After post hoc analysis for comparing patient groups with control group, PSVD was significantly reduced both in patients affected by moderate or severe NPDR (P=0.012) and in patients with PDR (P<0.001).
PDVD was 49.54%±11.01% in the control group and 44.57%±8.35% in patients affected by diabetes and without DR. Considering patients affected by DR, PDVD was 44.97%±8.10%, 34.75%±8.49% and 25.15%±6.67% in patients with mild NPDR, moderate or severe NPDR and PDR,respectively (P<0.001, ANOVA test; Figure 2; Table 1).
After post hoc analysis for comparing patients and healthy controls, deep vessel density was significantly reduced both in patients affected by mild NPDR (P=0.012) and in patients with moderate or severe NPDR (P<0.001) and with PDR (P<0.001;Table 1).
Two different testers measured vessel density and we were able to evaluate inter-observer variability in PRVD measurement(Figure 3). CCC was 0.834 (95%CI: 0.746-0.893) P<0.001 for superficial vessel density measurements and 0.890 (95%CI:0.828-0.930) P<0.001 for deep vessel density measurements indicating very good agreement between the investigators for the two tested measurements.
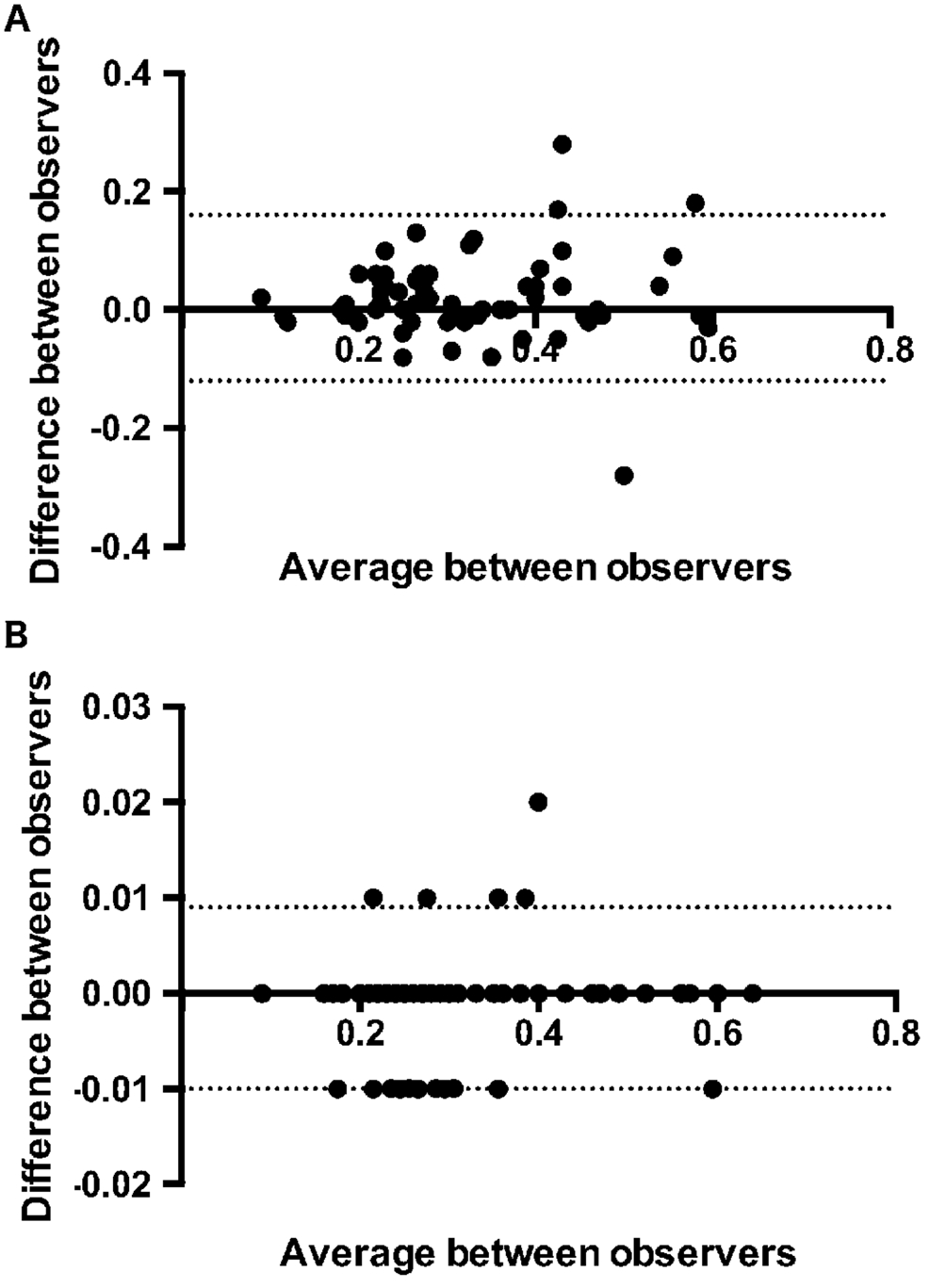
Figure 1 Bland-Altman plot of difference in FAZ area measurements between observers against their mean FA [panel A; mean difference 0.02 (95% limits of agreement -0.12; 0.16) ] OCT [panel B; mean difference -0.0007 (95% limits of agreement -0.01; 0.009)].
To identify if diabetic macular edema is a potential confounding factor in vessel density evaluation we performed a stratified analysis for the presence or absence of diabetic macular edema. No significant differences were found.
DISCUSSION
In this study, using OCTA, we investigated FAZ area and parafoveal superficial and deep vessel densities in diabetic patients, at different stages of DR. Overall, we found an increased FAZ area at increasing disease severity and both a reduction of parafoveal superficial and deep vascular density throughout the different stages of DR.
Ishibazawa et al[5] have already shown OCTA utility and sensibility in testing diabetic patients. Indeed, OCTA can clearly visualize microaneurysms and retinal non-perfused areas and quantitative information on new vessels can also be obtained.
Di et al[10] investigated the FAZ area using OCTA in diabetic patients with and without DR compared to control and found a significant increase of the FAZ area both in no DR patients(P=0.04) and DR patients with different grade of DR (P=0.00).An enlargement of the FAZ area both in the superficial and deep retinal plexuses in diabetic patients was also demonstrated by Freiberg et al[11] using OCTA.
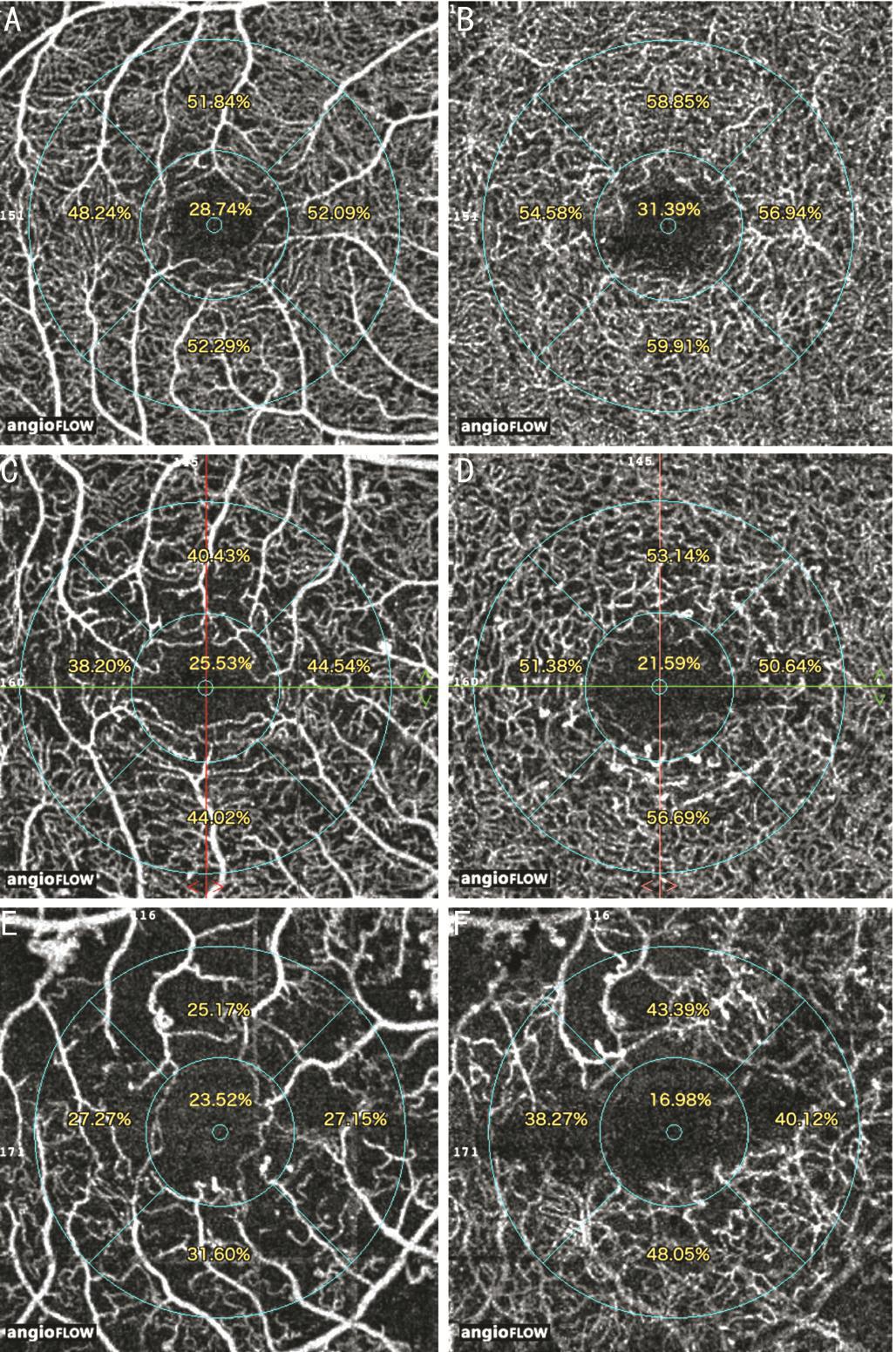
Figure 2 OCTA images of DR patients There is a reduction of parafoveal superficial and deep retinal vessel density in mild NPDR(A, B), severe NPDR (C, D) and PDR (E, F).
In our study the FAZ area evaluated by means of FA and OCTA showed a significant enlargement in moderate and severe NPDR group and PDR group compared to the control group. OCTA showed less inter observer variability in FAZ area assessment [CCC of 0.829 (95%CI: 0.736-0.891) P<0.001 for FA and 1.000 (95%CI: 0.999-1.000) P<0.001 for OCTA].The present study confirms what was already known from previous angiographic studies and reinforces recent acquisitions of OCTA studies focusing on variability of FAZ size in different stage of DR[10-17]. Moreover it demonstrates how OCTA is able to assess the status of the FAZ in relation to the degree of retinopathy without the need of dye and more accurately than the traditional angiography.
As previously reported by several authors using FA the increase of FAZ size is more significant in the later stages of retinopathy compared to mild or no retinopathy and this is possibly due to the presence of an interindividual variability in the size of the FAZ in normal subjects and in the early stages [12-17].Bresnik et al[15] and Mansour et al[17], were among the first to measure the area of the FAZ in FA, obtaining average values of about 0.231 mm2 to 0.280 mm2 in normal subjects up to values of 0.520 mm2 in advanced stages of retinopathy; the present study con firms the increase of FAZ area from normal controls to severe late stage of retinopathy with values ranging from 0.273±0.100 mm2 in normal controls, up to 0.380±0.111 mm2 in the PDR.
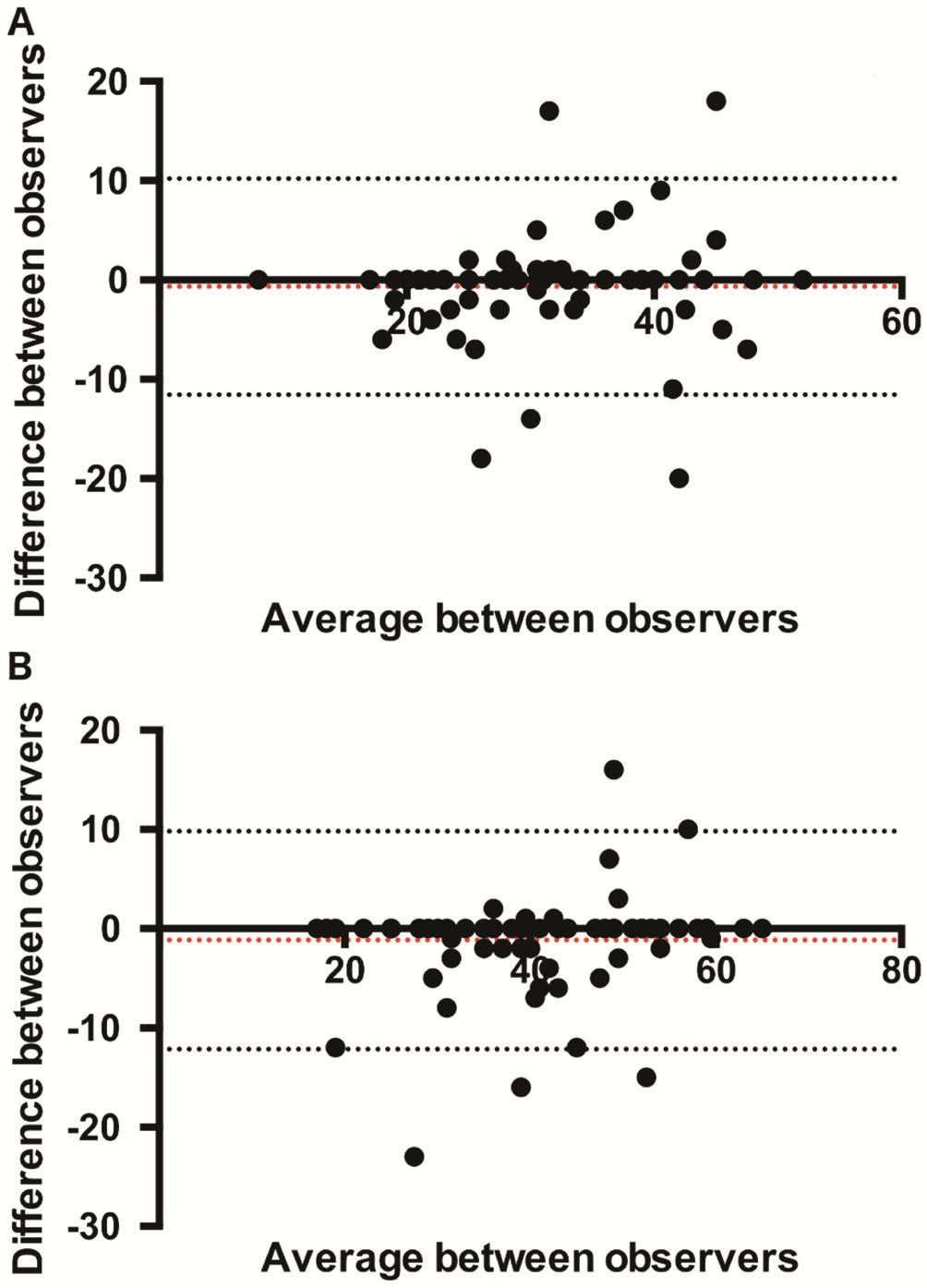
Figure 3 Bland-Altman plot of difference in superficial vessel density [panel A; mean difference -0.68 (95% limits of agreement-11.57; 10.20)] deep vessel density [panel B; mean difference -1.16(95% limits of agreement -12.15; 9.8)] measurements between observers against their mean.
Similarly to what was described by Di et al[10], in our study FAZ area measured with OCTA was significantly larger in DR patients compared to controls but it not differed between diabetic patients without retinopathy and normal controls. As already mentioned for FA measurements this can be related to the range of variation of FAZ area in no-DR group as it happens in normal controls[18].
OCT has been demonstrated a valuable imaging device to study both retinal and optic nerve head features[19-20]. In the last years, many authors have tried to characterize, by means of SD-OCT, additional vascular changes at different stages of DR, in addition to those identifiable on fundoscopy (e.g.microaneurysms and intraretinal microvascular abnormalities).These studies have been focused mainly on choroid alterations in diabetes and have shown choroidal thickening in patients affected by diabetes[21-22]. Interestingly, Kim et al[22] have demonstrated choroidal thickness increasing as the DR severity worsens.
Retinal vessels have been studied only in their superficial component in patients affected by diabetes. Several studies have shown diabetic individuals having early endothelial dysfunction, the latter leading to diminished arteriolar and venular vasodilator responses to light stimulus, compared with normal subjects[23-25]. Curiously, Lim et al[25] have shown vessel response to light stimulus diminishing as the DR worsens, the latter aspect suggests as the endothelial function lowering as the DR progresses.
Spaide et al[9] were the first to demonstrate in vivo in healthy subjects eyes by means of OCTA two distinct networks retinal capillaries, the superficial plexus and the deep plexus confirming what was already known from previous studies performed ex vivo with histology and immunohistochemistry.Distinct alterations of superficial and deep capillary plexuses were described by means of OCTA in retinal diseases such as macular telangiectasia and retinal ischemia associated to retinal artery occlusion[26-27].
Agemy et al[28] and coll showed by means of OCTA lower capillary perfusion density in nearly all layers of retinal capillary network in patients with DR compared with controls with a significant decrease in capillary perfusion density values as retinopathy progresses[28].
In our study a reduction of superficial capillary vessel density was observed in patients with moderate or severe NPDR(P=0.012) and in PDR patients compared to normal controls(P<0.001).
Deep capillary plexus vessel density was also significantly reduced both in patients affected by mild NPDR (P=0.012) and in patients with moderate or severe NPDR (P<0.001) and with PDR (P<0.001).
In the deep plexus the reduction of PRVD was present earlier being evident also in the mild NPDR as compared to the superficial plexus where it started from the moderate and severe NPDR and was more severe in the PDR. This is a new finding in DR and con firms what already suggested for other retinal vascular diseases concerning the primary involvement of the deep plexus in retinal vascular disorders[28].
Probably the evaluation of vessel density particularly in the deep plexus using OCTA could be used as a marker of disease severity also in the early stages of the disease.
A good agreement between two different investigators was detected in PRVD measurements both for superficial and deep plexus [CCC of 0.834 (95%CI: 0.746-0.893) P<0.001 for superficial vessel density measurements and 0.890 (95%CI:0.828-0.930) P<0.001 for deep vessel density measurements].In conclusion, in diabetic patients OCTA allows to detect FAZ area more accurately than FA with evidence of FAZ enlargement at increasing severity of DR. In addition OCTA showed good reproducibility in assessing superficial and deep plexus density with evidence of vessel density reduction earlier in the deep plexus compared to the superficial plexus in the most advanced stages of RD with more severe depletion of both plexuses at increasing disease severity.
ACKNOWLEDGEMENTS
Conflicts of Interest: Mastropasqua R, None; Toto L, None;Mastropasqua A, None; Aloia R, None; De Nicola C, None;Mattei PA, None; Di Marzio G, None; Di Nicola M, None;Di Antonio L, None.
REFERENCES
1 Klein R, Klein BE, Moss SE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XVI. The relationship of C-peptide to the incidence and progression of diabetic retinopathy. Diabetes 1995;44(7):796-801.
2 Song SJ, Wong TY. Currentconcepts in diabeticretinopathy. Diabetes Metab J 2014;38(6):416-425.
3 Gass JD. A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. IV. Diabetic retinal angiopathy. Arch Ophthalmol 1968;80(5):583-591.
4 Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, Zang E. Fluorescein angiography complication survey. Ophthalmology 1986;93(5):611-617.
5 Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K,Yokota H, Yoshida A. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol 2015;160:35-44.e1.
6 Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT; Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales.Ophthalmology 2003;110(9):1677-1682.
7 Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitude decorrelation angiography with optical coherence tomography. Opt Express 2012;20(4):4710-4725.
8 Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, Lu CD,Choi W, Fujimoto JG, Huang D. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012;3(12):3127-3137.
9 Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 2015;133(1):45-50.
10 Di G, Weihong Y, Xiao Z, Zhikun Y, Xuan Z, Yi Q, Fangtian D. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 2016;254(5):873-879.
11 Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S.Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2015;254(6):1051-1058.
12 Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y.Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina 2015;35(11):2377-2383.
13 Sander B, Larsen M, Engler C, Lund-Andersen H. Absence of foveal avascular zone demonstrated by laser scanning fluorescein angiography.Acta Ophthalmol (Copenh) 1994;72(5):550-552.
14 Sander B, Larsen M, Engler C, Lund-Andersen H, Parving HH. Early changes in diabetic retinopathy: capillary loss and blood-retina barrier permeability in relation to metabolic control. Acta Ophthalmol (Copenh)1994;72(5):553-559.
15 Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K.Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol 1984;102(9):1286-1293.
16 Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye (Lond)2005;19(3):322-326.
17 Mansour AM, Schachat A, Bodiford G, Haymond R. Foveal avascular zone in diabetes mellitus. Retina 1993;13(2):125-128.
18 Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveala vascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol 2016;100(5):671-676.
19 Carpineto P, Aharrh-Gnama A, Ciciarelli V, Mastropasqua A, Di Antonio L, Toto L. Reproducibility and repeatability of ganglion cellinner plexiform layer thickness measurements in healthy subjects.Ophthalmologica 2014;232(3):163-169.
20 Carpineto P, Nubile M, Toto L, Aharrh Gnama A, Marcucci L,Mastropasqua L, Ciancaglini M. Correlation in foveal thickness measurements between spectral-domain and time-domain optical coherence tomography in normal individuals. Eye (Lond) 2010;24(2):251-258.
21 Xu J, Xu L, Du KF, Shao L, Chen CX, Zhou JQ, Wang YX, You QS,Jonas JB, Wei WB. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology 2013;120(10):2023-2028.
22 Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci 2013;54(5):3378-3384
23 Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W, Wong TY. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care 2009;32(11):2075-2080.
24 Schiel R, Vilser W, Kovar F, Kramer G, Braun A, Stein G. Retinal vessel response to flicker light in children and adolescents with type 1 diabetes mellitus and overweight or obesity. Diabetes Res Clin Pract 2009;83(3):358-364.
25 Lim LS, Ling LH, Ong PG, Foulds W, Tai ES, Wong E, Lee SY, Wong D, Cheung CM, Wong TY. Dynamic responses in retinal vessel caliber with flicker light stimulation in eyes with diabetic retinopathy. Invest Ophthalmol Vis Sci 2014;55(8):5207-5213.
26 Yu S, Pang CE, Gong Y, Freund KB, Yannuzzi LA, Rahimy E, Lujan BJ, Tabandeh H, Cooney MJ, Sarraf D. The spectrum of superficial and deep capillary ischemia in retinal artery occlusion. Am J Ophthalmol 2015;159(1):53-63.e1-e2.
27 Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomographic angiography. JAMA Ophthalmol 2015;133(1):66-73.
28 Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG,Gentile RC, Hsiao YS, Zhou Q, Ko T, Rosen RB. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 2015;35(11):2353-2363.