Dear Editor,
I am Dr. David P Piñero from the Department of Optics,Pharmacology and Anatomy of the University of Alicante and from the Department of Ophthalmology of Vithas Medimar (Oftalmar) and Vithas Virgen del Carmen (Qvision)hospitals in Spain. I write to describe a case in which the visual acuity defocus curves (VADC) and contrast sensitivity defocus curves (CSDC) in photopic and mesopic vision after the implantation of a multifocal diffractive trifocal intraocular lens(IOL) are above average values in normal eyes. We discuss potential causes for this unexpected positive outcome.
Patients operated on with laser refractive surgery (LRS) in the past will develop with age presbyopia or age-related cataract. Possibly, these patients will desire to maintain their spectacle independence[1]. It is well known that LRS modifies the corneal shape and induces positive spherical aberration (SA) after myopic treatments and negative SA after hyperopic treatments[2]. For this reason, the use of some types of multifocal intraocular lenses (MIOLs) in such cases is controversial as these implants generate light scattering and deteriorate the optical quality of the eye. Indeed, poorer ocular optical quality is expected at far vision with MIOLs implanted in eyes with non-treated corneas compared to monofocal IOLs, with the potential of leading to some complaints, such as contrast sensitivity (CS) loss, glare, and halos[3]. Therefore,the visual performance with MIOLs may decrease even more after laser assisted in situ keratomileusis (LASIK), especially in mesopic conditions with low contrast tests[4]. However, the combination of the modified corneal shape after LRS and the specific optics of the MIOL may also generate an aberrometric compensation, allowing the patient to achieve visual acuities above 20/20 at far and near distances in mesopic conditions without a significant reduction of CS.
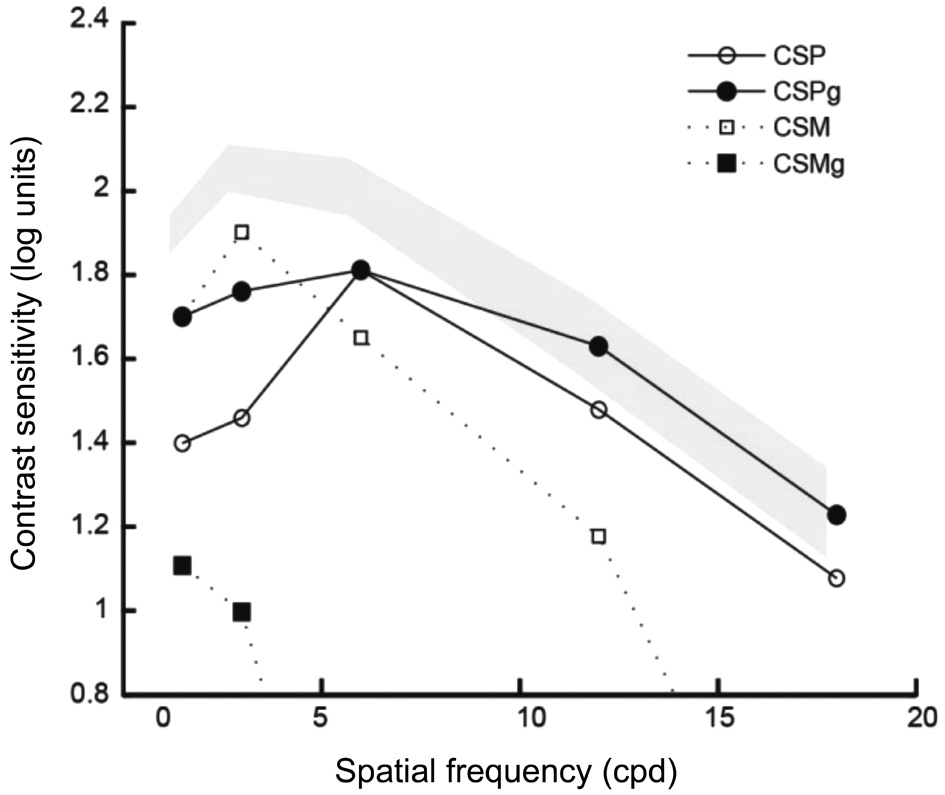
Figure 1 Contrast sensitivity function measured under photopic and mesopic conditions with (CSPg and CSMg) and without glare(CSP and CSM) The grey area represents the range of normality defined for post-LASIK patients without MIOLs implanted.
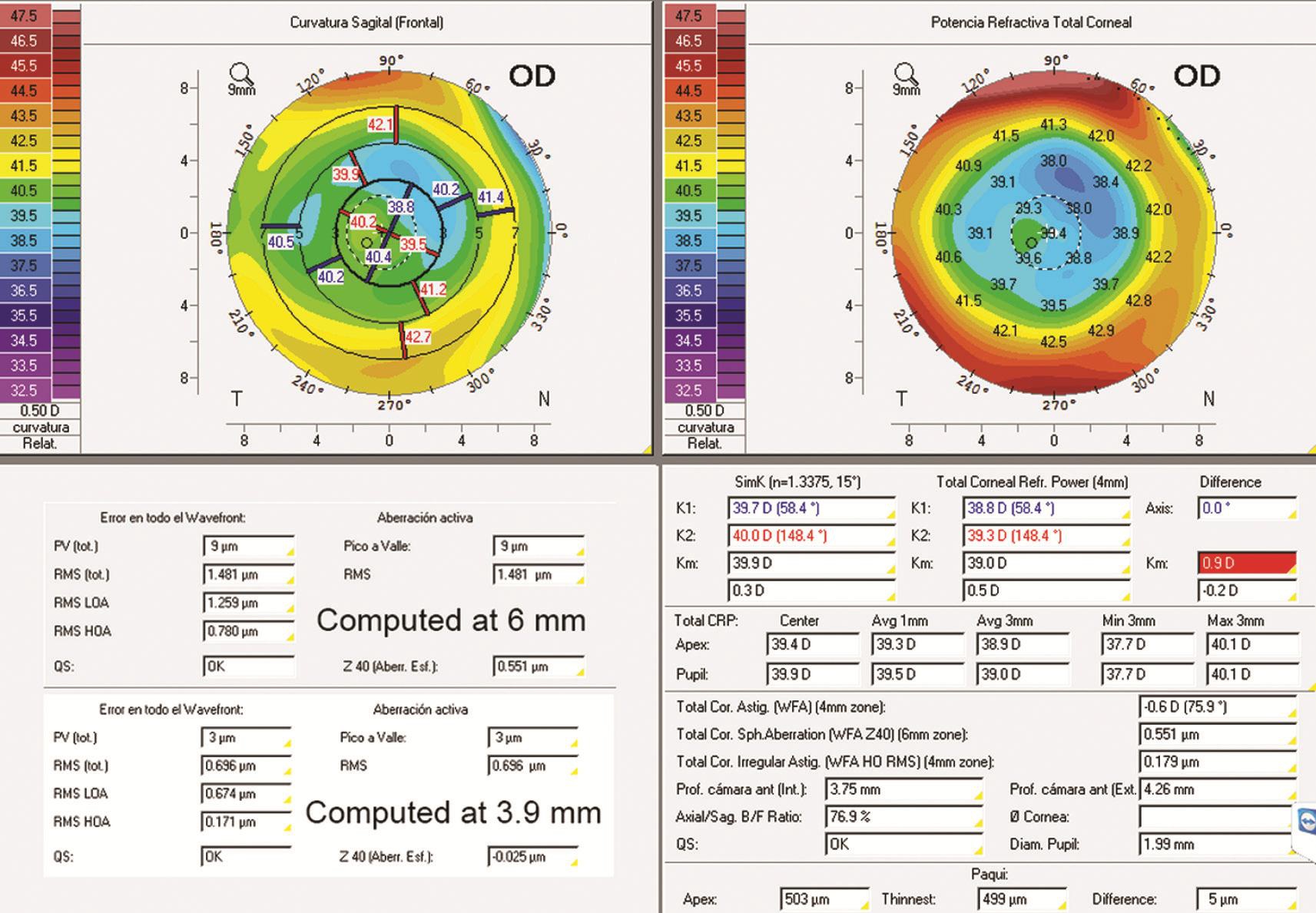
Figure 2 Images obtained from Pentacam at three months (two first rows).
A 48-year-old man that had been treated with LASIK five years ago for the correction of around 5 diopters (D) of myopia attended to our clinic seeking for surgical solution for his presbyopia. Refractive error was (-0.75) (-0.50) @80° in the right eye (RE) and (-1.75) (-0.50) @120° in the left eye (LE),with corrected distance visual acuity of -0.1 logMAR in both eyes. The near addition was +1.50 D. We used the Cataract Pre-op modulus of the Pentacam system (Oculus Optikgeräte,Wetzlar, Germany) for screening and IOL selection according to the four steps proposed by Maeda[5]. The corneal irregular astigmatism was 0.197 µm and corneal SA at 6 mm was 0.521 µm. This high positive SA might be partially compensated by a MIOL with negative SA, but not entirely. For this reason,we finally decided to implant the AT LISA Tri 839MPIOL(Carl Zeiss Meditec, Jena, Germany, -0.18 µm of SA)[6] in both eyes because the main desire of the patient was spectacle independence even though it was alerted that poorer vision might be presented at night in comparison with daylight conditions. The Haigis-L equation was used for computing the approximated IOL power, and the final IOL implanted was selected during surgery with the ORA system (Alcon Laboratories Inc.).
At one month after surgery, a residual refractive error of -0.25 D was found, achieving 0 logMAR at distance and J1 at near without correction. At three months postoperatively, VADC and CSDC were measured under photopic vision conditions with the Multifocal Lens Analyzer (MLA) for iPad[7]. The MLA was designed and programmed by Rodríguez-Vallejo et al[8-9] in order to measure automatically VADC by means of a crowded Snellen E that changes its size in 0.1 logMAR steps and determining the threshold through a staircase procedure for each one of the defocus lenses, from +1.00 D to -4.00 D in 0.50 D steps. Furthermore, CSDC can be measured with the same optotype and procedure, but maintaining a specific optotype size and modifying its contrast in 0.1 log unit steps[10]. In this case, the optotype size corresponded to a visual acuity of 0.3 logMAR[11]. Besides the automatic measurement of VADC and CSDC, we also measured the contrast sensitivity function (CSF) using the functional acuity contrast test (FACT,Vision Sciences Research Corporation, San Ramon, CA,USA) (Figure 1). As shown, the 6-month photopic CS values measured with and without glare for high spatial frequencies were within the range of normality defined for post-LASIK patients without MIOL implanted[12]. It is curious that there was no deterioration of CS for high spatial frequencies in this case in spite of being implanted with an MIOL that normally reduces CS. Likewise, VADCs were considerably better in comparison to the mean obtained in 19 eyes/subjects implanted with the same IOL and recorded in our database. VADC with a positive contrast optotype (i.e. higher letter luminance than background luminance) and CSDC were programmed for being performed at six months visit in mesopic conditions. At 6mo postoperatively, visual performance in mesopic conditions was as good as in photopic conditions at 3mo after surgery in spite of the significant amounts of corneal SA.
The pupil size and its dynamics were measured with the Keratograph 5M system (Oculus Optikgeräte, Wetzlar,Germany), obtaining a mean pupil value of 3 mm, oscillating between 1.9 and 3.9 mm under photopic and mesopic conditions, respectively. According to this, SA was recomputed for a 3.9-mm pupil using the Zernike analysis of the Pentacam system, resulting in a negative SA of only -0.025 µm (Figure 2).Although the SA for 6-mm pupil was very high, the CSDC measured under mesopic vision (Figure 3B) was not deteriorated because the pupil under these conditions was not large enough.Furthermore, another interesting finding is that VADC (Figure 3A) showed similar results for near (-0.1 logMAR, -3.0 D)and distance (-0.1 logMAR, 0 D) under mesopic conditions,although that the optical quality provided by the IOL was better at distance than at near[13]. This is because VADC was not very sensitive to small changes in ocular optical quality compared to CSDC (Figure 3B). CSDC was able to detect a considerable difference in visual performance between near and distance[9].
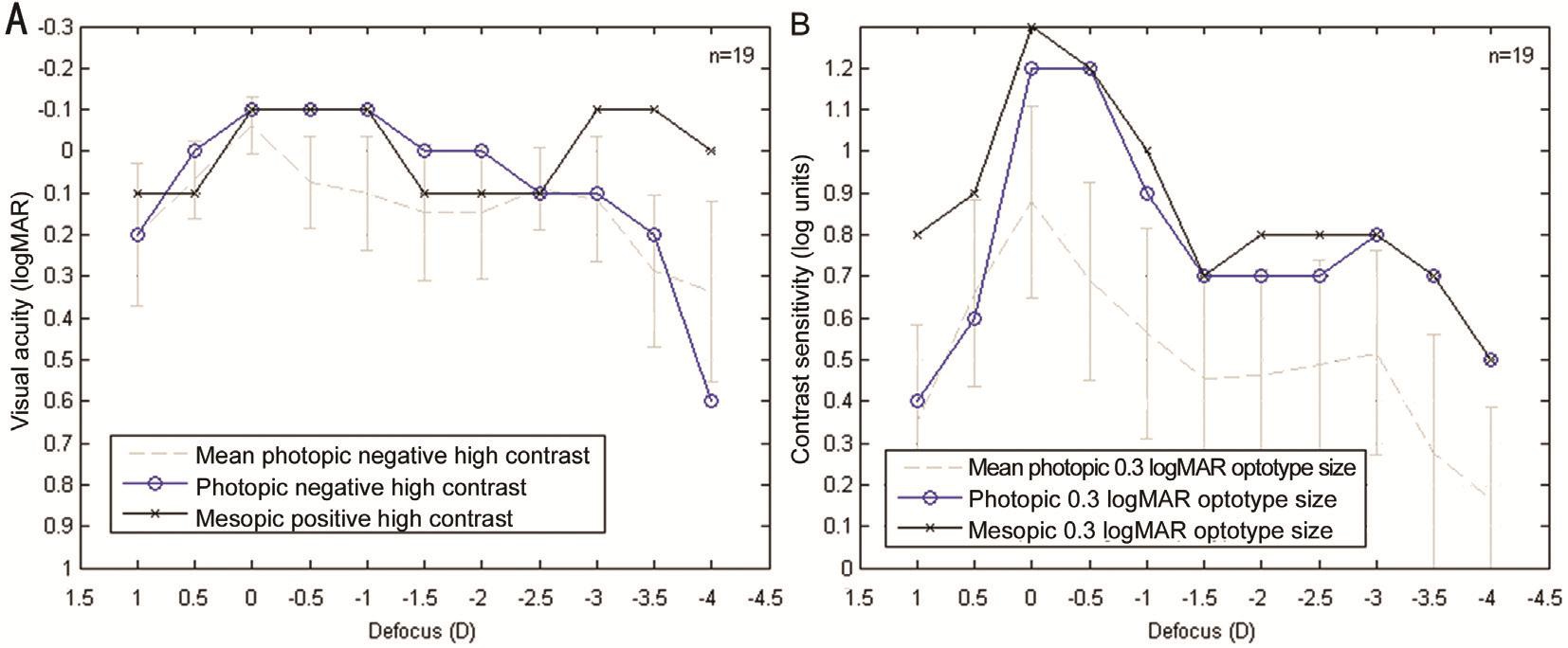
Figure 3 VADC (A) and CSDC (B) Photopic vision (blue) was measured at three months visit and mesopic vision (black) at six months visit only for the right eye. The results of this particular case are compared with the mean and standard deviation (grey) of a sample of 19 eyes/subjects implanted with the same IOL.

Figure 4 Image showing the perfect centration of the MIOL according to pupil center.
New devices are available for improving the characterization of the visual performance in patients implanted with MIOLs.In our protocol, Pentacam and Keratograph systems were used for taking clinical decisions and understanding the findings obtained in the case analyzed. Although some clinical guidelines are available in order to select the best IOL[5],the clinician should be prepared to modify these guidelines in some special cases. For instance, in this case, we have observed that the SA for a 6-mm pupil provided by the Catarat Pre-op modulus of Pentacam system was not the best indicator for selecting between spheric or aspheric IOLs as the patient’s pupil diameter under photopic and mesopic conditions was critical. The best option in a case such as that reported here is to recalculate corneal aberrations according to the maximum pupil size in mesopic vision.
Unfortunately, until very recently, there were no clinical tools for measuring automatically defocus curves under different light conditions for visual acuity and CS. Our research group developed a new application, MLA[14], that was incorporated in our clinical routine to analyze the visual performance of patients undergoing cataract surgery with implantation of MIOLs. We have obtained an excellent outcome in this patient,with levels of near and intermediate visual acuity that are even superior to those reported in previous studies evaluating the same MIOL in eyes with no previous LRS[15].
In summary, this clinical case shows that a visual performance above average under photopic and mesopic conditions (Figure 3)can be achieved in eyes with previous LRS treatment after MIOL implantation, such as in young patients with pupil size below the optical treatment zone size (Figure 2) and implanted with well-centered MIOLs (Figure 4). Therefore, patients with LRS are not necessarily bad candidates for MIOLs and a detailed study of the interaction between corneal aberrations and pupil dynamics must be done.
ACKNOWLEDGEMENTS
Conflicts of Interest: Fernández J, consultant for Oculus(Wetzlar, Germany) and Carl Zeiss Meditec (Jena, Germany);Rodríguez-Vallejo M, designer of Multifocal Lens Analyzer App and its distributor via Apple Store with his own developer account; Martínez J, None; Tauste A, None; Piñero DP,None.
REFERENCES
1 Black S. Successful restoration of visual acuity with an extended range of vision intraocular lens after multifocal laser ablation. Case Rep Ophthalmol 2016;7(3):193-197.
2 Muftuoglu O, Dao L, Mootha VV, Verity SM, Bowman RW, Cavanagh HD, McCulley JP. Apodized diffractive intraocular lens implantation after laser in situ keratomileusis with or without subsequent excimer laser enhancement. J Cataract Refract Surg 2010;36(11):1815-1821.
3 Gundersen KG, Makari S, Ostenstad S, Potvin R. Retreatments after multifocal intraocular lens implantation: an analysis. Clin Ophthalmol 2016;10:365-371.
4 Alfonso JF, Madrid-Costa D, Poo-López A, Montés-Micó R. Visual quality after diffractive intraocular lens implantation in eyes with previous myopic laser in situ keratomileusis. J Cataract Refract Surg 2008;34(11):1848-1854.
5 Maeda N. Assessment of corneal optical quality for premium IOLs with Pentacam. Highlights Ophthalmol 2011;39(4):2-5.
6 Carson D, Hill W, Hong X, Karakelle M. Optical bench performance of AcrySof® IQ ReSTOR®, AT LISA® tri, and FineVision® intraocular lenses.Clin Ophthalmol 2014;8:2105-2113.
7 Rodríguez-Vallejo M. Qvision Multifocal Lens Analyzer, for measuring Defocus Curves. Apple Store 2016. Availabled at: https://itunes.apple.com/us/app/qvision-multifocal-lens-analyzer.
8 Rodríguez-Vallejo M, Llorens-Quintana C, Furlan WD, Monsoriu JA. Visual acuity and contrast sensitivity screening with a new iPad application. Displays 2016;44:15-20.
9 Rodríguez-Vallejo M, Remón L, Monsoriu JA, Furlan WD. Designing a new test for contrast sensitivity function measurement with iPad. J Optom 2015;8(2):101-108.
10 Rodríguez-Vallejo M, Monsoriu JA, Furlan WD. Inter-display reproducibility of contrast sensitivity measurement with iPad. Optom Vis Sci 2016;93(12):1532-1536.
11 Holladay JT, Van Dijk H, Lang A, Portney V, Willis TR, Sun R,Oksman HC. Optical performance of multifocal intraocular lenses. J Cataract Refract Surg 1990;16(4):413-422.
12 Pesudovs K, Hazel CA, Doran RM, Elliott DB. The usefulness of Vistech and FACT contrasts sensitivity charts for cataract and refractive surgery outcomes research. Br J Ophthalmol 2004;88(1):11-16.
13 Gatinel D, Houbrechts Y. Comparison of bifocal and trifocal diffractive and refractive intraocular lenses using an optical bench. J Cataract Refract Surg 2013;39(7):1093-1099.
14 Fernández J, Rodríguez-Vallejo M, Martínez J. Bridging the gap between lab and clinical practice in assessing multifocal IOLs. Cataract Refract Surg Today 2016:1-3. Availabled at: http://crstodayeurope.com/articles/2016-feb/bridging-the-gap-between-lab-and-clinical-practice-inassessing-multifocal-iols/.
15 Mojzis P, Majerova K, Hrckova L, Piñero DP. Implantation of a diffractive trifocal intraocular lens: one-year follow-up. J Cataract Refract Surg 2015;41(8):1623-1630.