INTRODUCTION
Diabetes mellitus (DM) causes alterations in antioxidant activities, and there is emerging interest in oxidative stress causing a complex dysregulation of cell metabolism in the pathogenesis of diabetic vascular complications. Increased oxidative stress and decreased capacity of the antioxidative defense system have been demonstrated in the retina of diabetic animals[1-2]. Likewise, inflammatory responses in retinas of diabetic rodents have been shown by microarray analyses[3]. Additionally, it has been demonstrated that DM has led activation of transcriptional nuclear factor-kappa-B(NF-κB) in rodent retinas[4-5] and also caused the migration of the P65 subunit into the nuclei of retinal endothelial cells,pericytes, ganglion cells, or cells of the inner nuclear layer in rodent retina[6-8]. Besides, there is clear evidence that DM causes an increase of nitric oxide (NO), formed by the activity of endothelial nitric oxide synthase (eNOS), and it is one of the factors responsible for the pathogenesis of diabetic complications[9]. Laboratory measurement of NO is extremely difficult, hence other tissue indicators 8-hydroxy-2’-deoxyguanosine (8OHdG), malondialdehyde (MDA) for determining the cellular oxidant status have been studied biochemically. A deterioration in antioxidant enzyme activity[glutathione (GSH) redox system, superoxide dismutases(SOD), glutathione peroxidise (GSH-Px)] increases the susceptibility to oxidative stress; therefore, measurement of these enzymes have also been evaluated in the recent literature[10]. Accordingly, exogenous antioxidants have been used to decrease oxidative stress-dependent cellular alterations related with diabetes. Previous studies have demonstrated that certain fruits and vegetables having high levels of phenolic compounds are ideal sources of natural antioxidants[11-13].Consumption of pomegranate juice (PJ) with a high content of polyphenols decreases oxidative stress by stimulating the catalytic enzyme activities[11-15]. Pomegranate, in particular, condensed tannins and anthocyanins, are potent antioxidants which suppress NF-κB activating pro-inflammatory cytokines such as TNF, IL-1 and IL-12, and oxidative enzymes. Apart from its antioxidant capacity, anti-proliferative, anti-atherosclerotic and anti-inflammatory effect of PJ have been proposed[16-20].The present experimental study was designed to evaluate the potential antioxidant effect of PJ in retinas of diabetic rats.
MATERIALS AND METHODS
Pomegranate Processing Fresh pomegranates were washed,crushed, and then squeezed. It was treated enzymatically with pectinase to produce PJ and by-products. For improvement of extraction and filtration, α-1.4-galacturonide bonds were hydrolysed by pectinase. The juice was filtered, pasteurized,concentrated, and stored at -18℃[21-23]. Then dilution of 20 mL of concentrated juice in 500 mL of distilled water was acquired.The average of 2.5 mL diluted PJ includes 100 μL PJ, which is equivalent to 2.8 μmol total polyphenols per day.
Study Design This study was approved by the Scientific and Ethics Committee of animal care of Istanbul University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Twenty-eight adult Sprague-Dawley rats (230-250 g) were housed in cages under standard laboratory conditions with dark and light cycle.They were permitted free access to standard diet and water ad libitum. A single dose of intraperitoneally (i.p.) streptozotocin(STZ) (70 mg/kg STZ; Sigma, Deisenhofen, Germany)dissolved in 0.1 mol/L sodium citrate buffer with pH 4.5 was injected intraperitoneally[24]. NaCl 0.9% was injected i.p.only in the control group. Glucose levels were measured by glucometer, in blood samples obtained from the tail vein, 48h after the STZ injection. Blood glucose levels higher than 250 mg/dL were accepted as diabetic.
The animals were divided into four groups randomly: 1)control (CO); 2) control rats treated with pomegranate juice(PJ-CO); 3) diabetic rats (DM); 4) diabetic rats treated with pomegranate juice (PJ-DM). PJ-treated groups [PJ-CO (n=5),PJ-DM (n=9), respectively] were given 100 μL/day PJ via gastric gavage for a total of ten weeks, whereas the control and diabetes groups (n=5, n=9, respectively) received only 2.5 mL of saline. The total number of rats evaluated at the end was 27,because one of the diabetic rats died during the study.
The animals were anaesthetized with xylazine (10 mg/kg) and ketamine (100 mg/kg) and sacrificed by cervical dislocation after 10wk. The globes were enucleated, then the retinas were detached and used for DNA extraction or colorimetric analysis;these tissues were stored at -80℃ until the biochemical analyses.The other globes were immersed in 10% formaldehyde for histological analysis.
Biochemical Analyses The retina tissue was evaluated for oxidative DNA damage, lipid peroxidation products and antioxidant enzymes. 8OHdG in retina tissue was determined using NWLSS 8OHdG ELISA Kit, a competitive enzymelinked immunosorbent assay purchased from Northwest(Vancouver, WA, Canada) following the manufacturer’s instructions. It was necessary to extract and digest sample DNA prior to assay. Retina DNA was extracted using DNasy Blood and Tissue Kit (Qiagen), Spin-Column Protocol. This protocol is designed for purification of total DNA from animal tissues. Samples were assayed in the same day of enzymatic digestion. 8OHdG levels were expressed as 8OHdG pg/mg DNA.
All tissues were weighed and homogenized with 0.15M KCl solution and, 10% homogenates (w/v) of these tissues were prepared. First, tissue homogenates were centrifuged for 10min at 4℃ cold centrifuge 600 g. Then, it was centrifuged for 20min at 10 000 g and post-mitochondrial fraction was obtained. MDA and GSH levels were determined in tissue homogenates; SOD, GSH-Px activities were examined in the post-mitochondrial fraction of these homogenates. The bicinchoninic acid method was used for determination of the amount of protein in samples[25].
MDA, as an endpoint of lipid peroxidation, was calculated by detecting the absorbance of thiobarbituric acid reactive substances at 532 nm[26]. MDA levels were expressed as MDA nmol/mg protein. GSH levels were determined by using Elman reagent (5,5’-ditiobis-2-nitro benzoic acid)[27], and the results were expressed as GSH nmol/mg protein. SOD activity was measured by principle of increasing the ability of photooxidation rate in o-dianisidin sensitized with riboflavin[28].Colored product was measured spectrophotometrically at 460 nm, the results were expressed as U/mg protein, as specified. GSH-Px activity was measured according to the protocol of Lawrence et al[29]. The results were calculated using NADPH extinction coefficient and were expressed as nmol NADPH /mg protein /min.
Immunohistochemical Evaluation Eyesballs of sacrificed rats were fixed in 10% buffered formaldehyde for 12h.Dehydration, clearing, paraffin infiltration and embedding were alternated to all tissue samples. The paraffin bloks were cut into 3 microns sections by a microtome. The slides were stained with hematoxylin and eosin and examined under light microscopy. Briefly, sections were deparaffinized in xylene(2×5min) and rehydrated with successive 1min washes in 100%, 96%, 80%, and 70% ethanol. They were then stained with hematoxylin (2min), rinsed with distilled water, rinsed with 0.1% hydrochloric acid in 50% ethanol, rinsed with tap water for 15min, stained with eosin for 1min, and rinsed again with distilled water. The slides were then dehydrated with 95%and 100% ethanol successively followed by xylene (2×5min)and mounted with coverslips[30].
Table 1 The levels of 8OHdG, MDA, and activities of antioxidant enzymes GSH, SOD and GSH-Px of the four groups Mean±SD
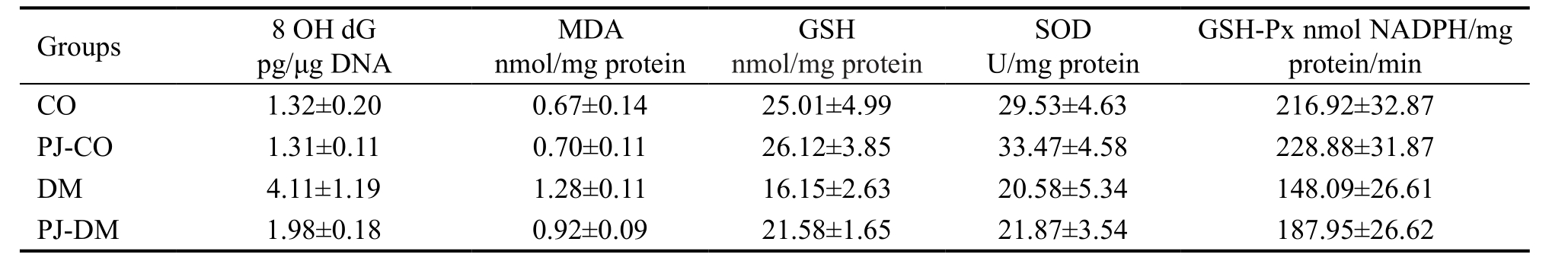
CO: Control group; PJ-CO: Pomegranate juice treated control group; DM: Diabetes mellitus group; PJ-DM: Pomegranate juice treated DM group.
Immunohistochemical study was performed with NOS2/ iNOS polyclonal antibody (Mybiosource) after antigen retrieval of paraffin-embedded tissue sections. Citrate buffered, pH 6.0 Avidin-Biotin-Peroxidase Complex (ABP) method and aminoethyl carbazole (AEC) chromogen were applied for immunohistochemical staining. The microscopic examination were performed by a single pathologist blinded to the study.
The staining of cytoplasmic eNOS (a surrogate for nitric oxide)and P65 (showing the activity of NF-κB) in the sensorial retina and choroid was evaluated, and the results were expressed as the percentage of retina and choroid cytoplasmically stained positive for eNOS and P65 in 1000 cells counted in the same section. The cases were evaluated for diffuseness and intensity of staining. According to staining diffuseness, sections were graded as follows: 0= no staining; 1= less than 25% staining;2= staining between 25% and 50%; 3= staining between 50%and 75%; 4= more than 75% staining. According to staining intensity, sections were graded as follows: 0= no staining;1= weak but detectable above control; 2= distinct; 3= intense staining. Immunohistochemical scores were obtained by adding diffuseness and intensity subscores[31].
Statistical Analysis Analyses were performed using IBM SPSS Statistics v.19. Biochemical parameters were compared by oneway-ANOVA and multiple comparisons were done with Tukey’s Post-hoc tests. The results of the histopathological and immunohistochemical evaluation of the groups were carried out by Pearson χ2 test. P<0.05 was accepted as statistically significant.
RESULTS
There was no significant difference in terms of body weight among the control and experimental groups (P=0.08). The levels of 8OHdG and MDA were significantly increased in the retina of the DM group compared to the CO group (P=0.001,P<0.001 respectively). Both 8OHdG and MDA levels were decreased in PJ-DM group compared to DM group (P=0.004,P<0.001 respectively). However, the levels of 8OHdG and MDA in PJ-DM group were still higher than the CO group(P=0.002, P=0.003 respectively) (Tables 1, 2).
The activities of antioxidant enzymes of SOD and GDH-Px were significantly decreased in the retina of the DM groupcompared to the CO group (P≤0.01; Tables 1, 2). The activity of antioxidant enzyme of GSH was also decreased, albeit this difference was not statistically significant (P=0.075;Tables 1, 2). The activities of all three antioxidant enzymes incresed with PJ intake both in the control, and the DM groups(Table 1). This increase was not statistically significant in the control group (P>0.05; Table 2). In the DM group, there was a statistically significant increase in the activity of GSH-Px(P=0.042; Table 2); whereas the increase in the activity levels of GSH, and SOD was not statistically significant (P=1, and P=0.938, respectively; Table 2). However, in PJ-DM group,the activities of both GSH and GSH-Px reached similar levels compared to the control group (P=0.744, P=0.314,respectively; Table 2), whereas the activity of SOD was still lower compared to the CO group (P=0.036; Table 2).
Table 2 Comparison of 8OHdG, MDA, GSH, SOD and GSH-Px between groups P1

CO: Control group; PJ-CO: Pomegranate juice treated control group;DM: Diabetes mellitus group; PJ-DM: Pomegranate juice treated DM group. 1Oneway-ANOVA, multiple comparisons were done with Post-hoc tests.

Figure 1 Normal control retina tissue.

Figure 2 P65 levels Intense staining by P65 immunohistochemistry of the diabetic retina tissue (A) and reduction of staining by P65 (B)immunohistochemistry of the diabetic retina tissue with pomegranate juice treatment. Magnification 100×, GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; IOS R&C: Inner and outer segments of rod sand cones; CHR: Choroid.

Figure 3 eNos levels Intense staining by eNOS immunohistochemistry of the diabetic retina tissue (magnification 40×) (A); Reduction of staining eNOS (B) immunohistochemistry of the diabetic retina tissue with pomegranate juice treatment (PJ-DM group, magnification 100×, GCL:Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; IOS R&C: Inner and outer segments of rods and cones; CHR: Choroid).
Table 3 Immunohistochemical staining scores
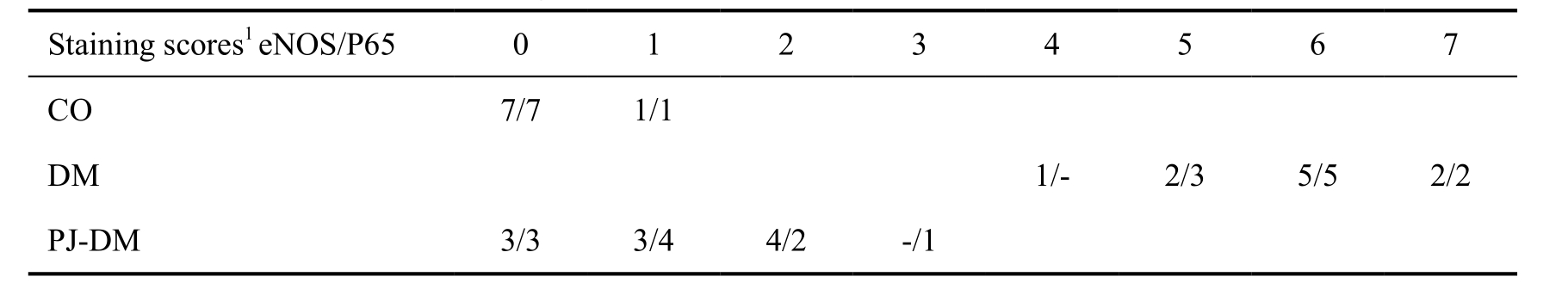
1Immunohistochemical staining scores were obtained by adding diffuseness and intensity subscores.
Immunohistochemical staining of the retina and thechoroid was evaluated (Figure 1), and it was found that the staining of eNOS and P65 was more intense in diabetic retinas than in those of the PJ-DM groups (P<0.001; Figures 2, 3). The intensity of staining of DM group diminished with PJ-treatment and approximated to the CO group (Table 3; Figures 2, 3).
DISCUSSION
The present study represented that lipid peroxidation measured as MDA and DNA oxidative damage evaluated by estimation of 8OHdG were significantly increased in the retinas of diabetic rats compared to CO group. Oxidative damage of cellular components mainly lipids, proteins and nucleic acids results in metabolic disturbances. In the literature, 8OHdG has been used as a key indicator of DNA oxidative damage and MDA as an indicator of lipid peroxidation[32-34]. There are a few reports showing the protective effect of PJ on DNA damage in the literature as well. In one of these, Faria et al[35]have demonstrated lower levels of pg 8OHdG/µg indicating oxidative damage of DNA in the liver of PJ treated mice.Similarly, we also found a reduction in the level of 8OHdG in retinas of PJ treated diabetic rats in our study.
Phenolic compounds of PJ are well known in inhibiting lipid oxidation and scavenging free radicals in vitro[36-37]. In this study, lower level of MDA was detected as an indicator of lipid peroxidation in PJ-DM group compared to DM group,consistent with the results of 8OHdG. Sohrab et al[38] recently reported in their randomized double-blind clinical trial that after 12wk of PC intake MDA decreased, total antioxidant capacity increased with a decrease in lipid peroxidation in adults with type 2 diabetes, concluding that PJ consumption may delay onset of diabetic complications related to oxidative stress. Besides, Shema-Didi et al[37] have found significantly lower levels of MDA in hemodialysis patients with one year of PJ intake in a randomized placebo-controlled trial.As a secondary endpoint, they concluded that prolonged PJ intake improves nontraditional cardiovascular risk factors,attenuates the progression of the atherosclerotic process,strengthens the innate immunity, and thus reduces morbidity among hemodialysis patients[37]. Yilmaz et al[39] reported that pomegranate extract decreased MDA, increased total antioxidant capacity, and decreased total oxidative stress in liver tissue, and remote organs of rats with experimental jaundice. In our experimental study we also found that there was a significant decrease in 8OHdG and MDA levels in PJDM group compared to DM group. Although the decreased levels of both of these parameters in PJ-DM group were still higher than the average level in control group, PJ intake decreased the oxidative stress biomarkers in diabetic rat retina tissues.
GSH and GSH-Px play important roles in preventing cellular damage caused by reactive oxygen species (ROS) such as free radicals and peroxides. SOD is specific for catalytic removal of superoxide and converts superoxide to hydrogen peroxide (H2O2). Correspondingly, Guo et al[32] have obtained an increase in GSH-Px levels and a decrease in SOD levels at final measurement in elderly subjects who were treated with PJ. In another experimental study by Çukurova et al[22],SOD levels were low in the diabetic lung and improved by PJ treatment. In a recent randomized, controlled study in patients with rheumatoid arthritis, pomegranate extract has shown to alleviate disease activity, and erythrocyte sedimentation rate as a biomarker of inflammation, and to increase GSH-Px concentration[40]. Ekhlasi et al[41] also reported that total antioxidant capacity increased in patients with non-alcoholic fatty liver disease who received PJ for 12wk in a randomized clinical trial. Consistent with these reports, we also noticed that GSH, SOD and, GSH-Px levels increased with PJ treatment both in the control and the diabetic groups, although this difference was statistically significant only with GSH-Px levels in diabetic rats. Moreover, the activities of both GSH and GSH-Px in PJ treated diabetic group increased to similar levels of the control group. These findings are compatible with the findings in the literature. Thus, recently, six weeks supplementation of PJ has been reported to increase total antioxidant capacity in patients with type 2 diabetes in a singleblind, randomized clinical trial[42]. Likewise, Shishehbor et al[43] found similar results with 4wk of concentrated PJ intake.
In literature, both NF-κB and eNOS are considered to play an important role in the pathogenesis of diabetic complications.Infact, these are two different pathways and the relationship between these pathways remains poorly understood. While some studies show that eNOS leads to the activation of NF-κB pathways[44-46], conversely in others eNOS inhibits NF-κB activation[47]. Besides, it has been demonstrated that NF-κB responsive miRNAs plays an important role in eNOS expression under inflammatory conditions[48-50]. For this reason,inhibition of NF-κB has been suggested as a new therapeutic intervention to improve endothelial dysfunction, by functional restoration of the eNOS/NO/cGMP pathway.
Inhibition of NF-κB has been suggested as a new therapeutic intervention to improve endothelial dysfunction underlying diabetic complications. In an experimental study, Eren et al[31] established the lung injury caused by NF-κB and eNOS activity in diabetic rats. Schubert et al[51] have shown that natural antioxidants like pomegranate wine can suppress NF-κB activation through a novel mechanism in vascular endothelial cells. Likewise, in our study staining of eNOS and P65 was found to be less intense in PJ treated diabetic retinas than those of the diabetes groups. Also, phosphorylated eNOS can be checked to ascertain decreased activity of eNOS in diabetic retina upon PJ treatment. Phosphorylated eNOS could not be studied in our study. However benefit of this decreased oxidative stress on the function of diabetic retinais still yet to be elucidated. Electrophsiological testing may be helpful in detecting these differences in diabetic, PJ treated, and control groups with further studies.
To the best of our knowledge, this is the first report about the evaluating the potential protective effect of PJ on retinal oxidative stress in diabetic rats. As a conclusion, in this study,assessment of biochemical oxidative markers revealed that prolonged PJ intake was found to decrease the oxidative stress via inhibition of lipid peroxidation and DNA oxidation. In addition, consistent with these results, immunohistochemical evaluation of the retina demonstrated less oxidative activity in PJ-DM group compared to that of the diabetic group.Further comprehensive studies are warranted to verify these experimental results.
ACKNOWLEDGEMENTS
We thank animal laboratory of Istanbul University, Cerrahpasa Medical Faculty.
Conflicts of Interest: Tugcu B, None; Nacaroglu SA, None;Gedikbasi A, None; Uhri M, None; Acar N, None; Ozdemir H, None.
REFERENCES
1 Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem 2002;80(5):771-779.
2 Ozdemir G, Ergün Y, Bakariş S, Kılınç M, Durdu H, Ganiyusufoğlu E. Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye (Lond) 2014;28(8):1020-1027.
3 Brucklacher RM, Patel KM, VanGuilder HD, Bixler GV, Barber AJ,Antonetti DA, Lin CM, LaNoue KF, Gardner TW, Bronson SK, Freeman WM. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Med Genomics 2008;1:26.
4 Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res 2003;37(11):1169-1180.
5 Zheng L, Szabó C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes 2004;53(11):2960-2967.
6 Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002;51(7):2241-2248.
7 Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA,Kern TS. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes.Diabetologia 2007;50(9):1987-1996.
8 Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy.Diabetes 2007;56(2):337-345.
9 Zheng L, Kern TS. Role of nitric oxide, superoxide, peroxynitrite and PARP in diabetic retinopathy. Front Biosci (Landmark Ed) 2009;14:3974-3987.
10 Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R, Jha KA, Srinivasan BP. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res 2014;125:193-202.
11 Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr 1998;128(12):2383-2390.
12 Ko SH, Choi SW, Ye SK, Cho BL, Kim HS, Chung MH. Comparison of the antioxidant activities of nine different fruits in human plasma. J Med Food 2005;8(1):41-46.
13 Verhagen H, Poulsen HE, Loft S, van Poppel G, Willems MI, van Bladeren PJ. Reduction of oxidative DNA-damage in humans by brussels sprouts. Carcinogenesis 1995;16(4):969-970.
14 Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol 2009;47(1):145-149.
15 Çam M, Hışıl Y, Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chemistry 2009;112(3):721-726.
16 El-Ashmawy NE, Khedr EG, El-Bahrawy HA, Abd El-Fattah EE.Effect of pomegranate hull extract on liver neoplastic changes in rats:more than an antioxidant. Nutr Cancer 2016;68(6):1044-1051.
17 Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 2000;48(10):4581-4589.
18 Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate)flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol 2006;44(7):984-993.
19 Modaeinama S, Abasi M, Abbasi MM, Jahanban-Esfahlan R. Anti tumoral properties of punica granatum (pomegranate) peel extract on different human cancer cells. Asian Pac J Cancer Prev 2015;16(14):5697-5701.
20 Singh RP, Chidambara Murthy KN, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 2002;50(1):81-86.
21 de Nigris F, Williams-Ignarro S, Lerman LO, Crimi E, Botti C,Mansueto G, D'Armiento FP, De Rosa G, Sica V, Ignarro LJ, Napoli C.Beneficial effects of pomegranate juice on oxidation-sensitive genes and endothelial nitric oxide synthase activity at sites of perturbed shear stress.Proc Natl Acad Sci U S A 2005;102(13):4896-4901.
22 Çukurova Z, Hergünsel O, Eren G, Gedikbaşi A, Uhri M, Demir G,Tekdöş Y. The effect of pomegranate juice on diabetes-related oxidative stress in rat lung. Turkiye Klinikleri J Med Sci 2012;32(2):444-452.
23 Kaplan M, Hayek T, Raz A, Coleman R, Dornfeld L, Vaya J, Aviram M. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J Nutr 2001;131(8):2082-2089.
24 Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 1976;193(4251):415-417.
25 Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH,Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC.Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150(1):76-85.
26 Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in enzymology 1978;52:302-310.
27 Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882-888.
28 Mylroie AA, Collins H, Umbles C, Kyle J. Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicol Appl Pharmacol 1986;82(3):512-520.
29 Lawrence RA, Burk RF. Glutathione peroxidase activity in seleniumdeficient rat liver. Biochem Biophys Res Commun 1976;71(4):952-958.
30 Hochstim CJ, Choi JY, Lowe D, Masood R, Rice DH. Biofilm detection with hematoxylin-eosin staining. Arch Otolaryngol Head Neck Surg 2010;136(5):453-456.
31 Eren G, Cukurova Z, Hergunsel O, Demir G, Kucur M, Uslu E, Dalo E,Uhri M, Tugcu V. Protective effect of the nuclear factor kappa B inhibitor pyrrolidine dithiocarbamate in lung injury in rats with streptozotocininduced diabetes. Respiration 2010;79(5):402-410.
32 Guo C, Wei J, Yang J, Xu J, Pang W, Jiang Y. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutr Res 2008;28(2):72-77.
33 Nekooeian AA, Eftekhari MH, Adibi S, Rajaeifard A. Effects of pomegranate seed oil on insulin release in rats with type 2 diabetes. Iran J Med Sci 2014;39(2):130-135.
34 Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' -deoxyguanosine(8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27(2):120-139.
35 Faria A, Monteiro R, Mateus N, Azevedo I, Calhau C. Effect of pomegranate (Punica granatum) juice intake on hepatic oxidative stress.Eur J Nutr 2007;46(5):271-278.
36 Rock W, Rosenblat M, Miller-Lotan R, Levy AP, Elias M, Aviram M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J Agric Food Chem 2008;56(18):8704-8713.
37 Shema-Didi L, Sela S, Ore L, Shapiro G, Geron R, Moshe G, Kristal B. One year of pomegranate juice intake decreases oxidative stress,inflammation, and incidence of infections in hemodialysis patients: a randomized placebo-controlled trial. Free Radic Biol Med 2012;53(2):297-304.
38 Sohrab G, Angoorani P, Tohidi M, Tabibi H, Kimiagar M, Nasrollahzadeh J. Pomegranate (Punicagranatum) juice decreases lipid peroxidation, but has no effect on plasma advanced glycated end-products in adults with type 2 diabetes: a randomized double-blind clinical trial. Food Nutr Res 2015;59:28551.
39 Yilmaz EE, Arikanoğlu Z, Turkoğlu A, Kiliç E, Yüksel H, Gümüş M.The protective effects of pomegranate on liver and remote organs caused by experimental obstructive jaundice model. Eur Rev Med Pharmacol Sci 2016;20(4):767-772.
40 Ghavipour M, Sotoudeh G, Tavakoli E, Mowla K, Hasanzadeh J,Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur J Clin Nutr 2017;71(1):92-96.
41 Ekhlasi G, Shidfar F, Agah S, Merat S, Hosseini AF. Effects of pomegranate and orange juice on antioxidant status in non-alcoholic fatty liver disease patients: a randomized clinical trial. Int J Vitam Nutr Res 2016:1-7.
42 Sohrab G, Ebrahimof S, Sotoudeh G, Neyestani TR, Angoorani P, Hedayati M, Siasi F. Effects of pomegranate juice consumption on oxidative stress in patients with type 2 diabetes: a single-blind,randomized clinical trial. Int J Food Sci Nutr 2017;68(2):249-255.
43 Shishehbor F, Mohammad shahi M, Zarei M, Saki A, Zakerkish M, Shirani F, Zare M. Effects of concentrated pomegranate juice on subclinical inflammation and cardiometabolic risk factors for type 2 diabetes: a quasi-experimental study. Int J Endocrinol Metab 2016;14(1):e33835.
44 Bell RM, Smith CC, Yellon DM. Nitric oxide as a mediator of delayed pharmacological (A1 receptor triggered) preconditioning; is eNOS masquerading as iNOS? Cardiovasc Res 2002;53(2):405-413.
45 Bolli R, Dawn B, Tang XL, Qiu Y, Ping P, Xuan YT, Jones WK,Takano H, Guo Y, Zhang J. The nitric oxide hypothesis of late preconditioning. Basic Res Cardiol 1998;93(5):325-338.
46 Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 2006;70(2):240-253.
47 Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 2006;38(10):1654-1661.
48 Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI, Fish JE, MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med 2013;5(7):1017-1034.
49 Tan G, Niu J, Shi Y, Ouyang H, Wu ZH. NF-κB-dependent microRNA-125b up-regulation promotes cell survival by targeting p38α upon ultraviolet radiation. J Biol Chem 2012;287(39):33036-33047.
50 Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol 2010;184(1):21-25.
51 Schubert SY, Neeman I, Resnick N. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J 2002;16(14):1931-1933.