INTRODUCTION
Retinopathy of prematurity (ROP) is a bilateral eye disease with abnormal retinal capillary development in premature infants. ROP is characterized by retinal ischemia,and neovascularization and proliferative retinopathy, which are the main contributing factors that limit the development of retinal vessels in premature infants[1]. ROP is a leading cause of childhood blindness, accounting for about 6% to 8% of cases[2]. The mechanisms of the pathogenesis of ROP have not been fully elucidated, but many studies suggest that retinal neovascularization and fibrosis play a leading role in the occurrence and development of ROP[3-4]. The development of ROP has been correlated to several factors,including angiogenic factors, cytokines and oxidative and neuroprotective growth factors[1]. In recent years, inflammation has also been reported to be involved in ROP[5-7].
Ratios of white blood cells (WBC) have been proposed as indicators for general inflammatory responses[8]. The neutrophil-to-lymphocyte ratio (NLR), lymphocyte-tomonocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR)have been proposed as potential markers of inflammation for predicting survival of patients in various diseases, including cancer, renal disease[9-12]. Ocular disorders, such as agerelated macular degeneration[13], diabetic retinopathy(DR)[14], have been reported to correlate with these WBC ratios, however the relationship between ROP and the NLR,LMR and PLR are not known. Therefore, the objective of this study was to evaluate the prognostic potential of LMR in patients with ROP. A retrospective cohort study was performed to assess the prognostic role of NLR, LMR and PLR on the clinical outcomes of patients with ROP.
SUBJECTS AND METHODS
Study Population Clinical data was collected from premature infants who underwent ROP screening in the First Affiliated Hospital of Nanchang University from January 2015 to December 2015. Infants without any other retinal disease and who gestational age less than 32wk or birth weight less than 2000 g were included in the study. Infants born with blood culture-proven sepsis, necrotizing enterocolitis and hematologic diseases, received a blood product transfusion or postnatal steroid therapy before the ROP screening were excluded. This study was approved by the medical Ethics Committee of the First Affiliated Hospital of Nanchang and adhered to the Declaration of Helsinki. Parents were informed of the study and provided written informed consent allowing their children to take part in this study.
Methods Fundus exams were performed on all infants according to the screening guidelines for ROP in China(2014). Initial screenings occured at 32wk postmenstrual age,or four to six weeks after birth. All exams were performed under mydriatic conditions by using two drops of tropicamide 0.5% and phenylephrine 2.5% before the examination began. Ophthalmological examinations were performed by an experienced ophthalmologist using a binocular indirect ophthalmoscope combined with sclera depressor and/or the RetCam III wide-angle digital retinal imaging system after topical anesthesia with proxymetacaine hydrochloride 0.5%eye drops. The ROP status of each infant was classified according to the international classification of ROP, including stage, zone, extent of disease, and presence or absence of plus disease[15]. Each infant was classified according to the maximum stage of ROP observed in either eye. Among the screened premature infants, 40 infants without ROP were randomly selected as the control group, and 40 infants with ROP were selected as the ROP group. Patients with threshold ROP, defined as 5 contiguous or 8 interrupted clock hours of stage 3 ROP with plus disease in zone I or II, or prethreshold type 1 ROP, defined as any ROP with plus disease or stage 3 without plus disease in zone I, and stage 2 or 3 with plus disease in zone II, were classified as severe ROP, who should be received treatment within 72h[16]. The other ROP infants were classified as non-severe ROP group. Other variables associated with ROP, such as birth weight, gestational age, sex, type of birth, and multiple pregnancies were also recorded. Patients with hypoxic-ischemic encephalopathy(HIE), premature rupture of membranes (PROM), respiratory distress syndrome (RDS), asphyxia neonatorum, and neonatal pneumonia were noted as have additional potential risk factors.Whole blood samples were collected within the first 24h of life, due to the potential need for blood transfusion later or the possibility of development of infection with or without sepsis.All blood samples were evaluated within the first 24h after birth. Peripheral venous blood (1 mL) was collected in tubes containing dipotassium ethylene diamine tetraacetate (EDTA-2K). Complete blood counts were evaluated by an automated hematology analyzer (Sysmex XE-2100, Kobe, Japan).
Statistical Analysis LMR was calculated by dividing the absolute lymphocyte count by the absolute monocyte count.Likewise, NLR and PLR were determined by dividing the absolute neutrophil count or the absolute platelet count by the absolute lymphocyte count, respectively. Continuous variables were presented as mean with standard deviation for normally distributed data or as medians and interquartile ranges (IQRs)for non-normally distributed data, and compared between ROP and non-ROP groups using one-way ANOVA test or Mann-Whitney nonparametric U test. Dichotomous variables were presented as absolute counts and percentage, and compared between groups by Chi-square statistical test. Univariate analysis was conducted to assess other potential risk factors for the presence of ROP, such as the NLR, LMR, PLR, HIE,PROM, RDS, asphyxia neonatorum and neonatal pneumonia.Logistic regression was used to estimate the significant independent risk factors associated with the presence of ROP.Exact P values <0.05 were considered statistically significant.The adjusted odds ratio (OR) and 95% confidence interval(CI) for each possible risk factor were calculated. Receiver operating characteristic (ROC) curve was plotted to determine the optimal cutoff value for LMR. All statistical analyses were performed using SPSS 22.0 (SPSS for Windows, version 22.0;SPSS, Inc., Chicago, IL, USA).
RESULTS
In this study, 80 preterm infants who met the inclusion criteria were enrolled. Their birth weight ranged from 650 to 1900 g,and gestational age ranged from 25 to 32wk. Of the 80 infants,40 presented some form of ROP. The distribution of stages of ROP was as follows: 18 (45%) developed stage 1 ROP; 10(25%), stage 2; and 12 (30%), stage 3; 15 patients (37.5%)had plus disease. The basic characteristics of premature infants in ROP group and non-ROP group are presented in Table 1. The mean birth weight was 1210±190 g (range, 650-1570 g) and 1393±260 g (range, 900-1900 g), respectively, and mean gestational age in ROP group and non-ROP group was 28.88±1.18wk (range, 25-31wk) and 29.70±1.18wk (range,28-32wk), respectively. Birth weight and gestational age were significantly different between both groups (P=0.001, 0.003,respectively). However, there were no statistically significant differences in terms of gender, type of birth, multiple pregnancy,WBC count, platelet count, neutrophil count, lymphocyte count, monocyte count, HIE, PROM, RDS, asphyxia neonatorum and neonatal pneumonia (P>0.05).
Table 1 Baseline clinical features and laboratory measurements of study subjects n (%)

GA: Gestational age; C/S: Caesarean section; NLR: Neutrophil-to-lymphocyte ratio; LMR: Lymphocyte-to-monocyte ratio; PLR: Platelet-to-lymphocyte ratio; HIE: Hypoxic-ischemic encephalopathy; PROM: Premature rupture of membranes; RDS: Respiratory distress syndrome. aP<0.05, statistically significant.
Table 2 Comparison of NLR and LMR values among groups
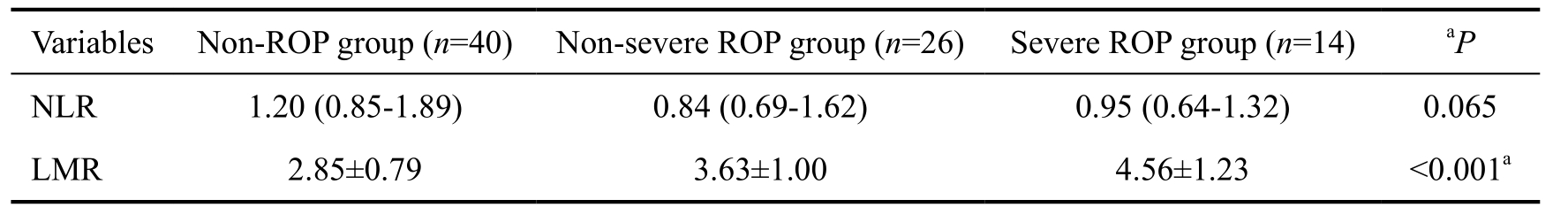
NLR: Neutrophil-to-lymphocyte ratio; LMR: Lymphocyte-to-monocyte ratio; ROP: Retinopathy of prematurity.aP<0.05, statistically significant.
The NLR values were significantly lower in patients with ROP compared to patients without ROP [median (IQR) 0.88(0.67-1.46) vs 1.20 (0.85-1.89); P=0.035]. Also, the LMR levels were significantly higher (P<0.001) in the ROP group(3.96±1.16) compared to the non-ROP group (2.85±0.79). The median PLR values were 61.99 (IQR, 50.23-75.98) in the ROP group and 69.24 (IQR, 55.52-88.12) in the non-ROP group, but the difference between groups was not statistically significant(P=0.104). Among the infants without ROP, the infants with non-severe ROP, and the infants with severe ROP who need treatment, the NLR values were not significant (P=0.065), but the LMR values in severe ROP group (4.56±1.23) and nonsevere ROP group (3.63±1.00) were, compared to those of non-ROP infants (2.85±0.79), significantly higher [P<0.001(one-way ANOVA); Table 2, Figure 1].
Logistic regression analysis suggested that independent risk factors for ROP were LMR (OR: 0.275; 95% CI: 0.134-0.564; P=0.001) and gestational age (Table 3). Although, birth weight between groups did not achieve statistical significance,P values approached significance (P=0.073) and may reach statistical significance with a larger sample size. Figure 2 shows that as an independent risk factor for ROP, the optimal cut-off value of LMR was 3.21, with 77.5% sensitivity and 70.0% specificity and an area under the ROC curve for LMR was 0.785 (95% CI: 0.683-0.887).
Table 3 Logistic regression analysis showing independent predictors of retinopathy
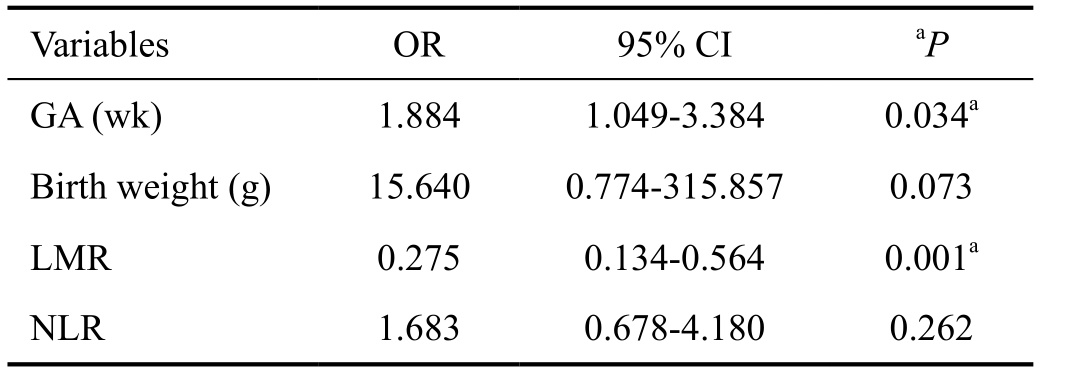
OR: Odds ratio; 95% CI: 95% confidence interal; GA: Gestational age; NLR: Neutrophil-to-lymphocyte ratio; LMR: Lymphocyte-tomonocyte ratio. aP<0.05, statistically significant.
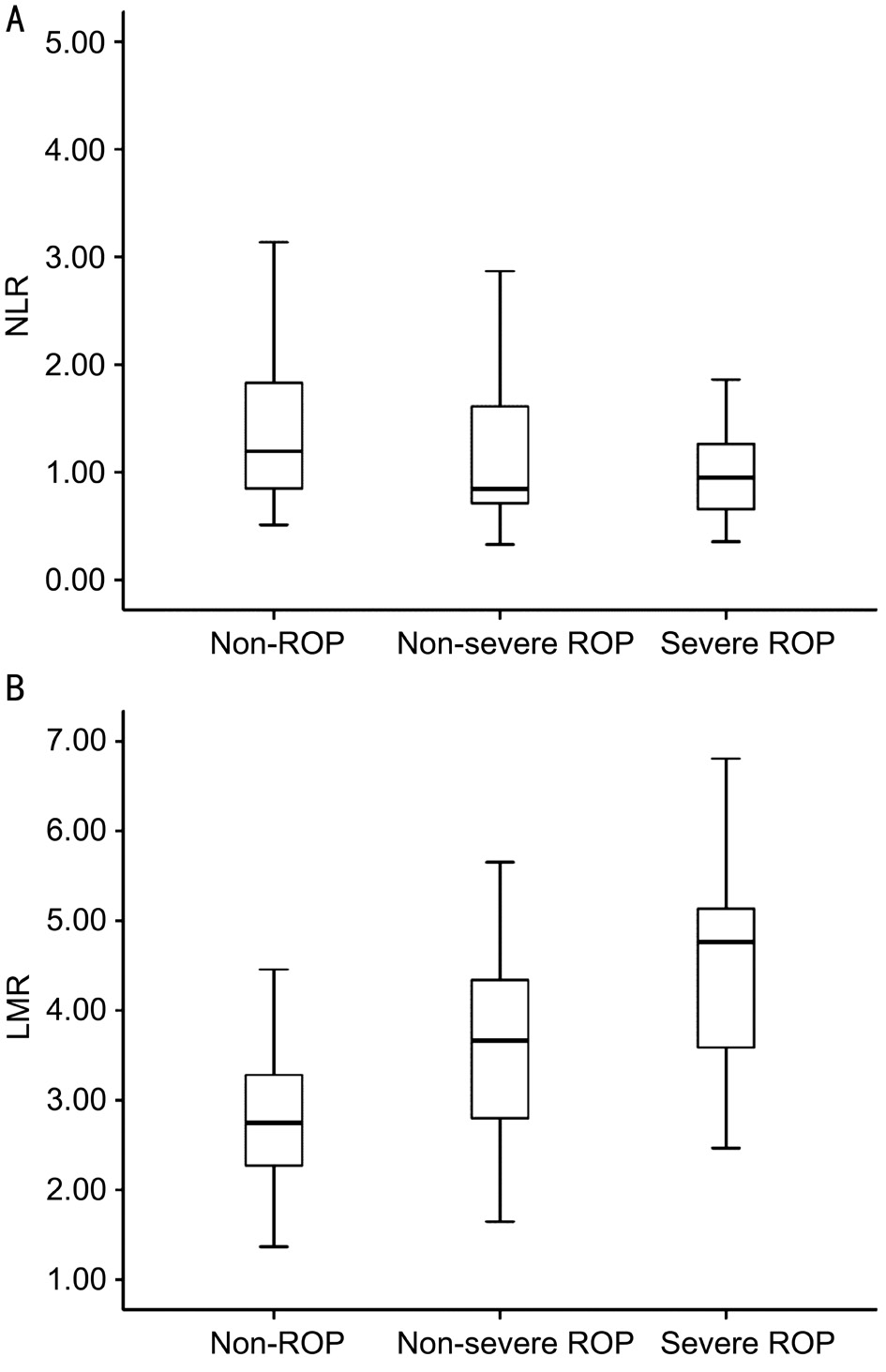
Figure 1 The boxplots showing the NLR and LMR levels in non-ROP, non-severe ROP, and severe ROP groups A: Comparison of the NLR levels among the three groups (P=0.065); B: Comparison of the LMR levels among the three groups (P<0.001).
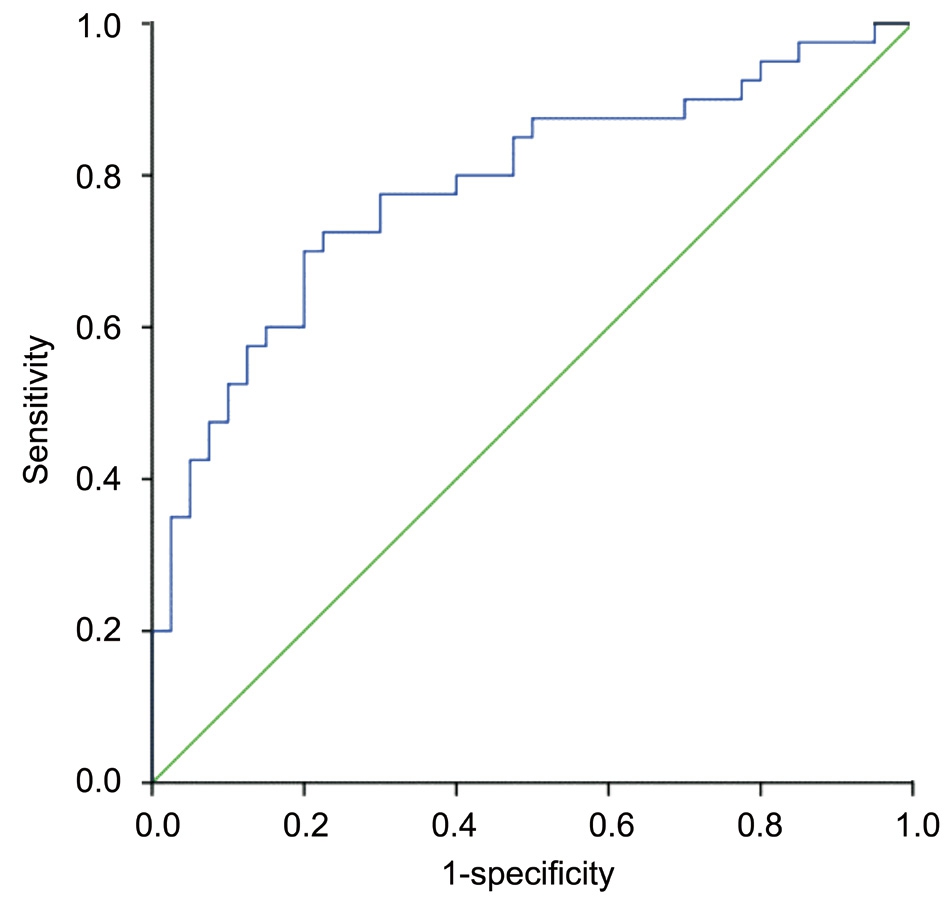
Figure 2 ROC curve analyses for LMR as a predictor of ROP.
DISCUSSION
It is known that the maternal systemic inflammatory responses and severe neonatal inflammatory reactions play a significant role in ROP pathogenesis[1,4]. Sood et al[7] have suggested that fetal inflammatory responses are an important factor in the cause of preterm birth. Woo et al[17] also reported that inflammatory mediators are significantly associated with the pathogenesis of ROP. Systemic inflammatory responses can directly or indirectly affect the formation of retinal neovascularization and increase the risk of ROP, independent of gestational age or severity of early systemic illness[5].These findings suggest an interaction between inflammatory responses and the pathogenesis of ROP.
In recent years, several novel inflammatory prognostic indicators derived from peripheral blood, such as the NLR,LMR, and PLR, have been widely investigated for their predictive prognostic value in tumors[11,18-19]. The use of NLR,which quantitates neutrophilia (an indicator of inflammation)and lymphopenia (an indicator of physiologic stress), is a valuable prognostic gage for evaluating patients with systemic inflammation[20]. NLR also reflects the balance between innate(neutrophils) and adaptive (lymphocytes) immune responses.Guthrie et al[21] suggest that the NLR may be more indicative of inflammation than total leukocyte count. In this study, the prognostic impact of the NLR on ROP was demonstrated on univariate analysis, but the significance of the association was lost with multivariate analysis (Tables 1, 3), which is similar to what has been reported by Kurtul et al[22]. However, the lymphocyte count alone turned out not to be an independent risk factor for ROP, which differs from the report of Kurtul et al[22]. One possible reason for this discrepancy is the case that the sample sizes in this study were slightly smaller.Additionally, subject heterogeneity may also account for this discrepancy.
Monocytes, an important component of peripheral blood,are considered an indicator of systemic inflammation, as monocytes are mobilization to migrate from the bone marrow to the peripheral blood[23]. LMR, as a novel inflammatory biomarker, reflects the balance between a favorable prognostic outcome involving lymphocytes and an unfavorable one,involving monocytes[24]. The prognostic value of LMR has been established in numerous tumor studies[24-26] and in retinal disease, such as DR[14], but has not been described in ROP. To the best of our knowledge, this is the first study to evaluate the association between LMR and ROP. In the present study,severe ROP patients are clearly inclined to get higher LMR compared to patients with non-severe ROP or without ROP,suggesting that there is an association between the LMR and ROP progression. Therefore, we hypothesized that the LMR of peripheral blood within 24h after birth is closely related to the occurrence and development of ROP and can be used as a reliable independent index for predicting ROP. Besides,the ROC curve was created to determine the cut-off value of LMR for the prediction of ROP, and figure out that the ideal value was 3.21. It suggests that the risk of ROP was higher in preterm infants with LMR over 3.21, but the significance needs to be verified by a large sample.
PLR is another indicator of systemic inflammation that has been validated as a prognostic predictor in some tumors[27-28].Recent studies have shown that platelets play an important role in angiogenesis, fibrin formation and deposition,platelet parameters, and changes in premature birth-related diseases, such as sepsis and RDS[29-30]. This is the first study that has specifically investigated the significance of PLR in ROP. However, PLR is not found to be associated with the development of ROP in this study, which may be due to the amount of the patient cohort. The mechanisms underlying the association between the PLR and ROP should be investigated in future studies.
The major limitation of this study is the small number of patients. Additionally, as a retrospective observational study,patients were not treated by the same doctor and it was difficult to ensure the consistency of the clinicopathological data. Finally, all patients enrolled in this study were Chinese which provides little data to understand the influence of ethnic diversity. Further investigations including more patients are needed to investigate the possible role of serum LMR and NLR levels in ROP disease progression. Moreover, to better understand the prognostic role of the LMR and NLR,additional investigations should be conducted in these ratios,such as the differences based on gestational age, the dynamic changes in ROP progression, the relationships with different treatments and so on.
In conclusion, the current study revealed that higher LMR,measured within a critical window of 24h of birth, is independently associated with ROP. Early detection of abnormal LMR levels may be helpful for predicting the development of ROP in premature neonates. Additionally,NLR and PLR are uncorrelated with the development of ROP in premature infants. However, these findings should be validated in large-scale prospective studies.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Foundation of China (No.81360151; No.81760179); Natural Science Foundation of Jiangxi Province (No.20171BAB205046);Jiangxi Province Education Department Key Foundation (No.GJJ160033); Technology and Science Foundation of Jiangxi Province (No.20141BBG70027); Jiangxi Province Education Department Scientific Research Foundation (No.GJJ13147);Health Development Planning Commission Science Foundation of Jiangxi Province (No.20141031); Nanchang University Postgraduate Case Construction Project (No.0902-0210210802).
Conflicts of Interest: Hu YX, None; Xu XX, None; Shao Y,None; Yuan GL, None; Mei F, None; Zhou Q, None; Cheng Y, None; Wang J, None; Wu XR, None.
REFERENCES
1 Hellström A, Smith LE, Dammann O. Retinopathy of prematurity.Lancet 2013;382(9902):1445-1457.
2 Courtright P, Hutchinson AK, Lewallen S. Visual impairment in children in middle- and lower-income countries. Arch Dis Child 2011;96(12):1129-1134.
3 Cavallaro G, Filippi L, Bagnoli P, La Marca G, Cristofori G, Raffaeli G,Padrini L, Araimo G, Fumagalli M, Groppo M, Dal Monte M, Osnaghi S, Fiorini P, Mosca F. The pathophysiology of retinopathy of prematurity:an update of previous and recent knowledge. Acta Ophthalmol 2014;92(1):2-20.
4 Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr 2011;23(2):173-178.
5 Tremblay S, Miloudi K, Chaychi S, Favret S, Binet F, Polosa A,Lachapelle P, Chemtob S, Sapieha P. Systemic inflammation perturbs developmental retinal angiogenesis and neuroretinal function. Invest Ophthalmol Vis Sci 2013;54(13):8125-8139.
6 Lee J, Dammann O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med 2012;17(1):26-29.
7 Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K,Hougaard D, Shankaran S, Carlo W. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 2010;67(4):394-400.
8 Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR,Renlund DG, Muhlestein JB. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45(10):1638-1643.
9 Song YJ, Wang LX, Hong YQ, Lu ZH, Tong Q, Fang XZ, Tan J.Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumor Biol 2016;37(4):5285-5293.
10 Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F,Gao T, Zhang L, Wang S. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med 2015;13:66.
11 Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X,Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients.Med Oncol 2014;31(12):305.
12 Turkmen K, Ozcicek F, Ozcicek A, Akbas EM, Erdur FM, Tonbul HZ.The relationship between neutrophil-to-lymphocyte ratio and vascular calcification in end-stage renal disease patients. Hemodial Int 2014;18(1):47-53.
13 Ilhan N, Daglioglu MC, Ilhan O, Coskun M, Tuzcu EA, Kahraman H, Keskin U. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm 2015;23(4):287-290.14 Yue S, Zhang J, Wu J, Teng W, Liu L, Chen L. Use of the monocyte-tolymphocyte ratio to predict diabetic retinopathy. Int J Environ Res Public Health 2015;12(8):10009-10019.
15 International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123(7):991-999.
16 Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006;117(2):572-576.
17 Woo SJ, Park KH, Lee SY, Ahn SJ, Ahn J, Park KH, Oh KJ, Ryu A.The relationship between cord blood cytokine levels and perinatal factors and retinopathy of prematurity: a gestational age-matched case-control study. Invest Ophthalmol Vis Sci 2013;54(5):3434-3439.
18 Song X, Zhang GM, Ma XC, Luo L, Li B, Chai DY, Sun LJ.Comparison of preoperative neutrophil-lymphocyte, lymphocytemonocyte, and platelet-lymphocyte ratios in patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Onco Targets Ther 2016;9:1399-1407.
19 Yamagishi T, Fujimoto N, Nishi H, Miyamoto Y, Hara N, Asano M,Fuchimoto Y, Wada S, Kitamura K, Ozaki S, Kishimoto T. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 2015;90(1):111-117.
20 Ozgonul C, Sertoglu E, Gokce G. Accurate use of neutrophil/lymphocyte ratio in patients with keratoconus. Cornea 2015;34(2):e4-e5.
21 Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC,Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88(1):218-230.
22 Kurtul BE, Kabatas EU, Zenciroglu A, Ozer PA, Ertugrul GT, Beken S, Okumus N. Serum neutrophil-to-lymphocyte ratio in retinopathy of prematurity. J AAPOS 2015;19(4):327-331.
23 Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11(11):762-774.
24 Li J, Jiang R, Liu WS, Liu Q, Xu M, Feng QS, Chen LZ, Bei JX, Chen MY, Zeng YX. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One 2013;8(12):e83069.
25 Eo WK, Kwon S, Koh SB, Kim MJ, Ji YI, Lee JY, Suh DS, Kim KH,Kim HY. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of endometrial cancer. J Cancer 2016;7(5):538-545.
26 Go SI, Kim RB, Song HN, Kang MH, Lee US, Choi HJ, Lee SJ, Cho YJ, Jeong YY, Kim HC, Lee JD, Kim SH, Kang JH, Ling H, Lee GW.Prognostic significance of the lymphocyte-to-monocyte ratio in patients with small cell lung cancer. Med Oncol 2014;31(12):323.
27 Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ, Lee JH. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17(3):216-222.
28 Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13(7):499-503.
29 Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17(1):47-58.
30 Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organismspecific response? Pediatrics 2003;111(6 Pt 1):1411-1415.