INTRODUCTION
Subjective or manifest refraction (MR) is the goldstandard method to determine the eye’s refractive status.Subjective refraction requires trained optometrists and results in a time-consuming procedure. Automated refraction (AR) is fast and does not need special skills from the operator, making it widely used in clinical practice. However, AR has not been able to substitute MR and it is normally used just to estimate the starting point for MR. The accuracy of AR decreases in some circumstances, including previous corneal refractive surgery, media opacities, and multifocal intraocular lenses(IOLs)[1-4].
The Lentis Mplus X multifocal IOL (Oculentis GmbH) is a single-piece radially asymmetric refractive multifocal IOL with a near vision segment of +3.00 diopters (D). The light is refracted to the near focus in a specific sector, and the rest of the IOL acts as a monofocal lens for far vision. The Lentis Mplus X multifocal IOL has a very different design in comparison with previous refractive multifocal IOLs that used annular concentric areas of alternating refractive powers.
The current study was designed to assess the repeatability of AR in patients implanted with Lentis Mplus X multifocal IOL,and to determine whether AR is sufficiently accurate to be useful as a starting point for MR. To the best of our knowledge,there are no previous studies addressing this issue.
SUBJECTS AND METHODS
Study Design This prospective study included patients who had cataract or refractive lens exchange surgery with implantation of the spherical Lentis Mplus X multifocal IOL,and potential decimal visual acuity (VA) of at least 20/40.Patients with corneal astigmatism were not excluded, and they were informed to probably need a secondary ablative procedure to achieve emmetropia. Only one eye per patient was selected for the study[5-7]. Exclusion criteria were ocular diseases other than cataract, and photopic pupil size lower than 3.5 mm. Patients were informed about the details of the study, written informed consent was obtained before surgery in accordance with the Declaration of Helsinki, and institutional review board approval was obtained from the hospital Ethics Committee.
A complete ophthalmological examination was performed in all subjects before surgery, including MR, potential VAmeasured with a potential acuity meter, pupil diameter at photopic conditions (90 cd/m2) measured with a Colvard pupilometer, corneal topography, optical biometry (IOL Master, Carl Zeiss Meditec), slit lamp examination, macular optic coherence tomography (OCT, Topcon), and fundoscopy.The power of the IOL was calculated with the SRK/T formula for eyes with axial length equal or longer than 22 mm and with the Holladay II formula for eyes with axial length lower than 22 mm. All eyes were targeted for emmetropia, with A-constant of 118.5.
Table 1 Descriptive statistics (mean, standard deviation, maximum and minimum values) obtained after surgery, both for refraction, and VA

UDVA: Uncorrected distance visual acuity; UNVA: Uncorrected near visual acuity at 40 cm; DCVA:Distance corrected visual acuity; DCNVA: Distance corrected near visual acuity at 40 cm.
Surgeries were performed by two experienced surgeons(Munoz G and Rohrweck S). Phacoemulsification using a 2.75 mm clear-corneal incision placed on the steepest meridian,capsulorhexis, and symmetric implantation of the Lentis Mplus X multifocal IOL in the capsular bag with the near sector at 6 o’clock position were performed in all eyes.
Patients were examined 1d, 1wk, 1 and 3mo after surgery,with data collection for the study corresponding to the last 3mo visit. Subjective refraction was performed aiming for the maximum positive spherical power yielding to the best VA. To avoid focus error when performing MR, the three-step method previously published was used[2-3]. Before MR, three AR measurements were obtained for each eye with the KR-8000 autorefractor (Topcon), which has shown proper agreement with MR[8]. At the same time, three automated keratometry readings (KR) were obtained with the same device.
After AR and MR were obtained under normal physiological conditions, patients were dilated with 10% phenylephrine drops, and AR measurements were repeated to check for possible differences under pupil dilation.
IOL centration was assessed by slit lamp examination under dilated conditions, and by retinoscopy under physiological pupil conditions.
Statistical Analysis Manifest and automated refractions in conventional script [sphere (S), cylinder (C), and axis (j)],were converted into power vectors coordinates (M, J0, J45) by the following formulas, according to Thibos and Horner[9].

SigmaPlot v.11 for Windows was used for statistical analysis.Three consecutive AR measurements were averaged (after power vector conversion) before and after dilation with phenylephrine 10%. One-sample t-test was used to check for differences between the means of the 3 AR measurements in each eye (non-dilated and dilated) compared with the initial AR non-dilated measurement, to study the repeatability of AR measurements.
Regression plots were constructed for S, and for each of the power vector components (M, J0 and J45), and correlation coefficients were obtained. Normality of data was evaluated by the Shapiro-Wilk test prior to any hypothesis testing. Paired t-tests were performed to look for differences in the components of refraction between the AR and MR measurements; the Wilcoxon Signed Rank test was used when data failed to pass the normality test. Agreement was evaluated by Bland-Altman plots created with Medcalc v.12.5 software for Windows,plotting the difference between AR and MR vs MR; intraclass correlation coefficients were also calculated. Automated KR was compared to manifest astigmatism by means of the astigmatic components of power vectors (J0 and J45).
Assuming 0.25 D as the minimum difference with clinical significance between AR and MR, and considering 0.62 as the expected standard deviation of change based in a previous study[10], the sample size was estimated as n=67 for a statistical power of 1-β=0.9.
RESULTS
Ninety patients (90 eyes) completed the follow-up period of 3mo. Two patients were excluded because of IOL decentration and were analyzed separately. One patient was excluded because early posterior capsule opacification and 1 patient was excluded because of cystoid macular edema.
Thus, data were collected from 86 eyes of 86 patients (35 men and 51 women). The mean age of the patients was 63.3±6.9y(SD) (range 49 to 80y). Mean pupil size under photopic conditions was 4.7±0.8 mm (range 3.5 to 6.5 mm). Table 1 shows descriptive statistics for the sample, regarding manifest refraction and VA after surgery.
Repeatability of Automated Refraction Measurements The mean differences between the initial nondilated AR measurement and the mean of the 3 measurements before and after dilation were not statistically significant (1-sample t-test,P>0.05), suggesting the initial measurement was similar to the means of the 3 AR measurements both nondilated and dilated(Table 2). For that reason, initial nondilated AR measurements were used in all subsequent analyses.
Agreement Between Automated Refraction and Manifest Refraction Measurements The mean value of the difference between AR and MR was -1.28±0.29 D for sphere (Wilcoxon Signed Rank test, P<0.001), and -1.41±0.39 D for spherical equivalent (Paired t-test, P<0.001) (Figure 1).
Intraclass correlation coefficient was 0.96 for sphere, and 0.93 for spherical equivalent.
The mean value of the difference between the AR cylinder and the MR cylinder was 0.02±0.26 D for J0 (Wilcoxon Signed Rank test, P=0.432), and 0.14±0.31 D for J45 (Wilcoxon Signed Rank test, P<0.001). Intraclass correlation coefficient was 0.68 for J0, and 0.44 for J45.
Figure 2 shows the Bland-Altman plots for sphere, spherical equivalent and astigmatism. Sphere showed the best agreement,with no defined pattern of data distribution. Astigmatic components of refraction showed a wide pattern of vertical distribution of data, reflecting the wide range of values for the difference between AR and MR for a given MR value(especially for MR=0).
Agreement Between Automated Keratometry and Manifest Refraction Measurements The mean value of the difference between automated KR and the MR cylinder was 0.02±0.17 D for J0 (Wilcoxon Signed Rank test, P=0.20), and 0.03±0.17 D for J45 (Wilcoxon Signed Rank test, P=0.18) (Figure 3).
Intraclass correlation coeficient was 0.91 for J0 and 0.80 for J45.The Bland-Altman plots comparing automated KR and MR for both components of astigmatism are shown in Figure 4.
Decentered Lentis Mplus X Multifocal Intraocular Lens Lentis Mplus X multifocal IOL decentration was detected in 2 eyes (2.2%) (Table 3).
Lens decentration appeared early, within the first week of follow-up, and remained stable thereafter. The defocus curve of these 2 eyes resembled that of a monofocal IOL. Interestingly,AR and MR showed no differences in sphere in these 2 eyes. When Lentis Mplus X multifocal IOL was decentered superiorly, both AR and MR was around -3.00 D of spherical equivalent; when the IOL was decentered inferiorly, both AR and MR showed spherical equivalent around emmetropia.
DISCUSSION
This prospective study evaluated the repeatability and accuracy of AR after spherical Lentis Mplus X multifocal IOL implantation. There are several methods to approximate the refractive status after lens extraction with IOL implantation[11-14]including keratometry, retinoscopy and AR, but MR is still considered the gold standard and the rest of techniques are normally used to determine a starting point. The popularity of autorefractors is attributable to their availability and ease of use. However, autorefraction’s accuracy decreases in the presence of a multifocal IOL[1-4].
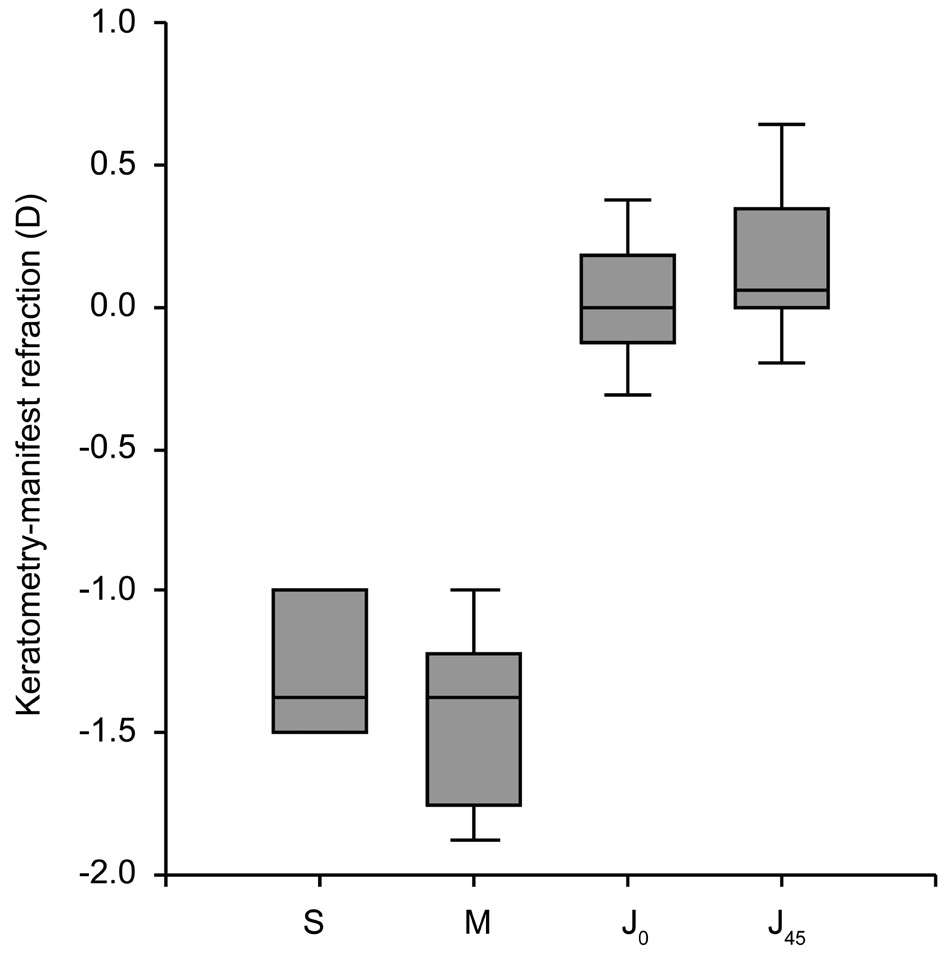
Figure 1 Difference between automated refraction and subjective refraction for S (sphere), M (spherical equivalent), and J0 and J45(vector components of astigmatism).
Table 2 Repeatability of automated refraction: mean values(±standard deviations) for the difference between the initial AR measurement and the mean of 3 AR measurements
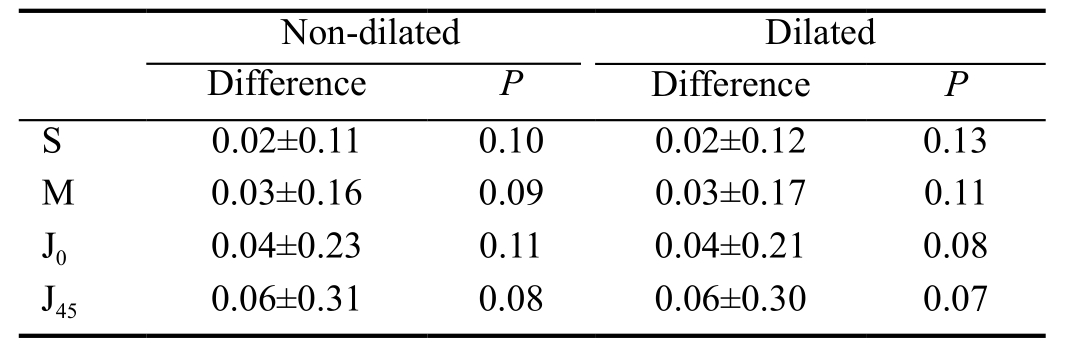
Table 3 Patients excluded from the analysis because of intraocular lens decentration

AR: Automated refraction; MR: Manifest refraction.
The present study demonstrated the good repeatability of AR both for sphere and astigmatism in the presence of a Lentis Mplus X multifocal IOL, as previously reported for other refractive multifocal IOLs[10]. Regarding accuracy, AR showed a pseudomyopia or trend toward more negative values for sphere, with a mean difference AR-MR of around -1.25 D.Although sphere showed statistically significant differences between AR and MR, the correlation was high (R2=0.85),meaning that AR sphere is useful to determine where to start MR in the presence of a Lentis Mplus X multifocal IOL,taking into account this scale factor of -1.25 D. In a previous paper with the toric multifocal LS-313 MF IOL, the difference between AR and MR sphere was anecdotally reported to be -1.25 D also[15]. In the work by van der Linden et al[1], a similar difference between AR and MR for sphere of around 1 D was found, but those authors stated in their work that AR measurements seems not to be a valid starting point for MR. We disagree with that point, since we have demonstrated a good repeatability for AR measurements, with a high correlation between AR and MR, so AR can be considered as a good starting point for MR, just having into account a +1.25 D factor to be added to the AR result. Regarding astigmatism,the cylinder components showed lower mean differences(0.02 D for J0 and 0.14 D for J45), but correlation between AR astigmatism and MR astigmatism was low (R2=0.27 and 0.11, respectively), making AR astigmatism useless in clinical practice.
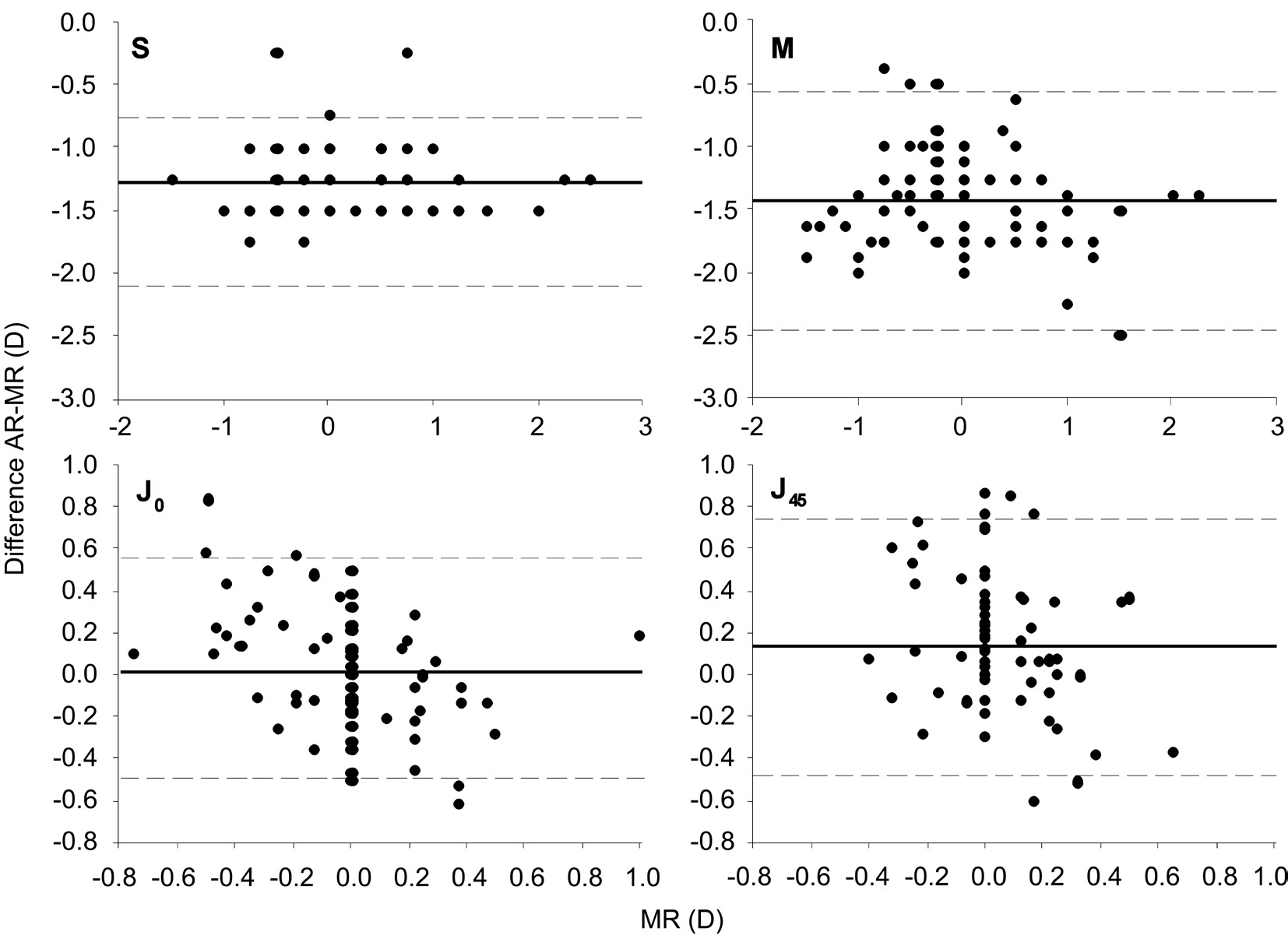
Figure 2 Agreement (Bland-Altman plot) between the difference automated refraction minus manual refraction (AR-MR) and manual refraction (MR) for S (sphere), M (spherical equivalent), J0 and J45 (vector components of astigmatism).
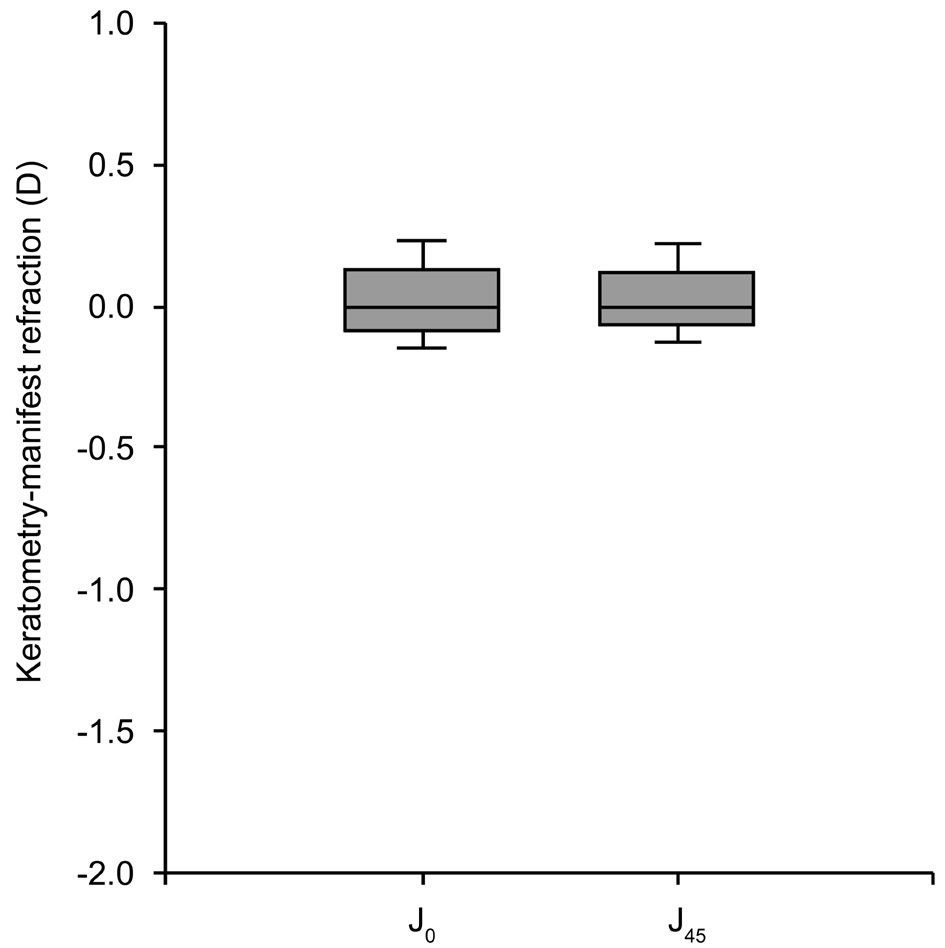
Figure 3 Difference between automated keratometry and subjective astigmatism refraction for J0 and J45 (vector components of astigmatism).
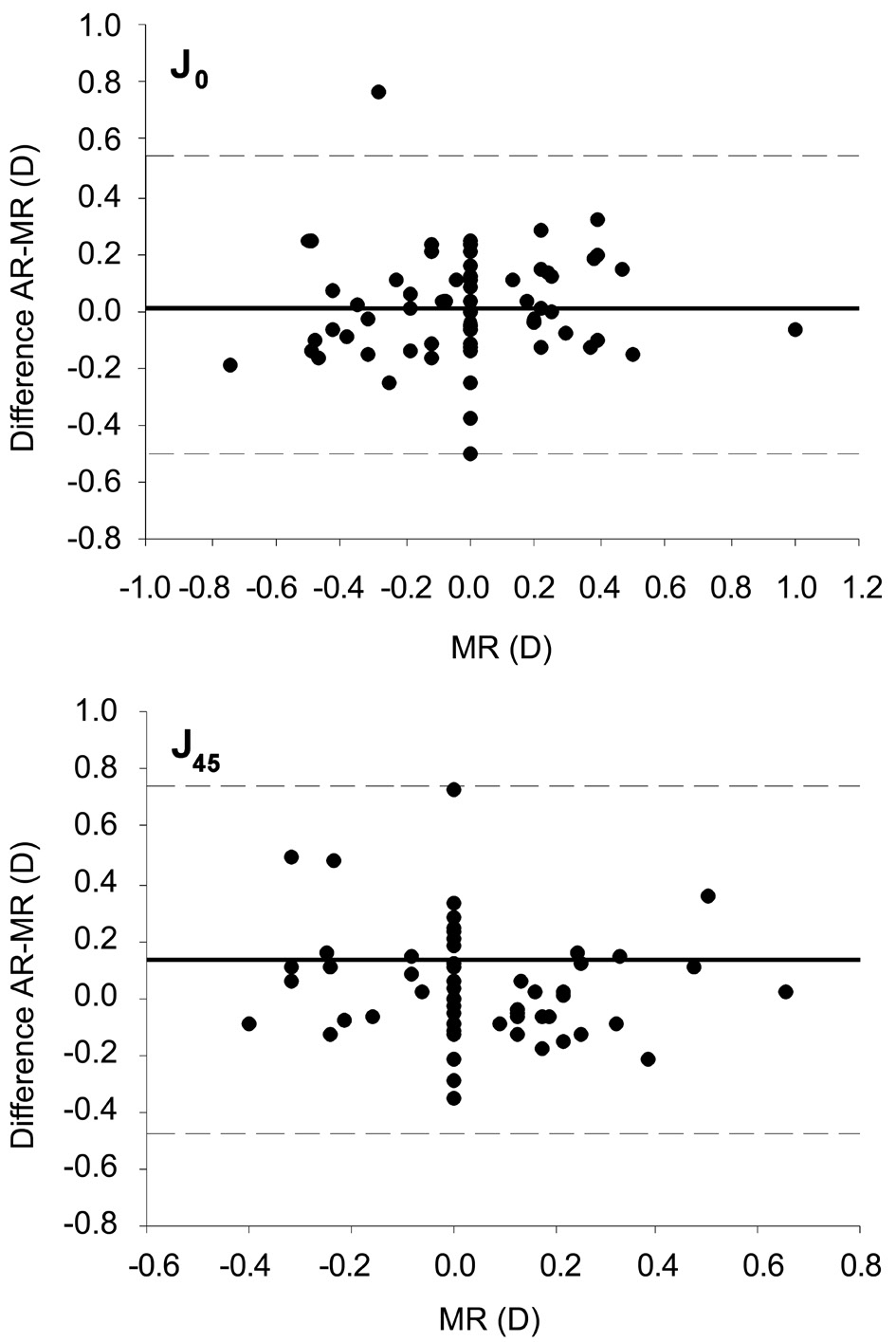
Figure 4 Agreement (Bland-Altman plot) between the difference automated keratometry minus manual refraction astigmatism(KR-MR) and manual refraction astigmatism (MR) for J0 and J45(vector components of astigmatism).
Pseudomyopia with AR has been previously reported with other refractive multifocal IOLs[1]. It can be explained by the way in which AR works and considering the especial design of the Lentis Mplus X multifocal IOL, with two different refractive surfaces and a difference between them of 2.50 D at spectacle plane. Autorefractors give an average of the two refractions yielded by the two foci of the IOL. Thus, for an emmetropic eye implanted with a Lentis Mplus X multifocal IOL, the eye has two refractions: 0.00 D (far focus) and -2.50 D(near focus), resulting in an average of -1.25 D which approximates the mean difference found in the present study between AR and MR.
The present study also showed better agreement between KR and MR astigmatism than between AR and MR astigmatism.However, some amounts of corneal astigmatism were perfectly tolerated by emmetropic Lentis Mplus X multifocal IOL eyes with no differences in VA when performing MR. The same happened for low amounts of AR myopia. This phenomenon was referred to as “defocus tolerance” of the Lentis Mplus X multifocal IOL and has been previously explained showing the defocus curve of the IOL with a wide hump of the peak of VA corresponding to the far focus[16-17]. The defocus tolerance of the Lentis Mplus X multifocal IOL may represent several advantages including better tolerance to low ametropia and less need for secondary enhancement procedures. However,defocus tolerance can make MR less accurate in the presence of objective (AR or KR) low degrees of ametropia such as small amounts of astigmatism. For instance, it can be difficult to determine the more exact MR of an eye with AR and KR astigmatism of 0.50 D, without improvement or worsening on MR with such refraction due to the defocus tolerance of the lens.
One limitation of the study is that the results have been obtained with a specific autorefractor model, but we have observed (unpublished data) the same results with other autorefractors as well. Other possible limitation lies in the fact that we have only evaluated spherical IOLs, whereas the Lentis MPlus multifocal IOL has a toric platform[18], and further studies must confirm our results with the toric geometry.
Although retinoscopy is superior to AR in the general population[19], we found it a difficult technique in eyes implanted with the Lentis Mplus multifocal IOL (any of the Mplus models) because of the presence of two opposite retinoscopy shadows, especially in small pupils. In our experience the two major utilities of retinoscopy in this kind of patients are: first, to recognize the implant of a Lentis MPlus multifocal IOL in a pseudophakic eye, given the characteristic retinoscopic shadow movements observed with this IOL, and second to give the clinician a quick idea about the centration of the IOL, estimating the part of the reflex belonging to the far and near foci, by exploring the horizontal meridian with the retinoscopy streak in vertical orientation. If the Lentis MPlus multifocal IOL is positions with the near segment in other direction[20], retinoscopy could lead to scissors reflexes until steak is aligned with the proper meridians, consistent with the placement of the near segment.
In our experience, significant decentration carried the loss of the multifocal properties of the Lentis Mplus X multifocal IOL. When the lens was significantly decentered, the autorefractor only measured one part of the IOL as if the lens was monofocal. In the 2 eyes with decentered IOLs AR was more coincident with MR. One eye showed a superior IOL decentration, so that the inferior segment of the Lentis Mplus X multifocal IOL was occupying the pupillary area, making the patient myopic of -2.75 D in MR and around -3.00 D in AR. The other patient had an inferior IOL decentration, with the far part of the IOL lying in the pupillary area, with AR sphere similar to the MR, and near to emmetropia.
In summary, AR measurements in eyes implanted with a Lentis Mplus X multifocal IOL were useful for sphere, with a correcting factor of approximately -1.25 D, but not for cylinder.Automated KR showed better accuracy and correlation with MR than AR astigmatism. In clinical practice, we suggest AR sphere plus 1.25 D and the KR cylinder as a starting point for MR in the presence of a Lentis Mplus X multifocal IOL. If AR measurements were equal to MR, decentration of the IOL should be suspected.
ACKONWLEDGEMENTS
Conflicts of Interest: Albarrán-Diego C, None; Muñoz G,None; Rohrweck S, None; García-Lázaro S, None; Albero JR, None.
REFERENCES
1 van der Linden JW, Vrijman V, Al-Saady R, van der Meulen IJ, Mourits MP, Lapid-Gortzak R. Autorefraction versus subjective refraction in a radially asymmetric multifocal intraocular lens. Acta Ophthalmol 2014;92(8):764-768.
2 Albarrán-Diego C, Muñoz G, Ferrer-Blasco T. Subjective refraction before LASIK enhancement in bioptics procedures with refractive multifocal intraocular lenses. J Refract Surg 2011;27(8):556-557.
3 Albarrán-Diego C, Muñoz G, Ferrer-Blasco T, Garcia-Lazaro S.Prevention of hyperopic surprise after LASIK in patients with refractive multifocal intraocular lenses. Eur J Ophthalmol 2011;21(6):826-829.
4 Muñoz G, Albarrán-Diego C, Sakla HF. Autorefraction after multifocal IOLs. Ophthalmology 2007;114(11):2100.
5 Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt 2013;33(1):7-14.
6 Armstrong RA, Davies LN, Dunne MC, Gilmartin B. Statistical guidelines for clinical studies of human vision. Ophthalmic Physiol Opt 2011;31(2):123-136.
7 McAlinden C, Khadka J, Pesudovs K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol Opt 2011;31(4):330-338.
8 Hashemi H, Khabazkhoob M, Asharlous A, Soroush S, Yekta A, Dadbin N, Fotouhi A. Cycloplegic autorefraction versus subjective refraction: the Tehran Eye Study. Br J Ophthalmol 2016;100(8):1122-1127.
9 Micó V, Albarrán-Diego C, Thibos LN. Power vectors for the management of astigmatism: from theoretical to clinical applications. Buckley R.Astigmatism: Types, Diagnosis and Treatment Options. New York: Nova Science Publishers 2014:27-80.
10 Muñoz G, Albarrán-Diego C, Sakla HF. Validity of autorefraction after cataract surgery with multifocal ReZoom intraocular lens implantation. J Cataract Refract Surg 2007;33(9):1573-1578.
11 Goss DA, Grosvenor T. Reliability of refraction-a literature review. J Am Optom Assoc 1996;67(10):619-630.
12 Mao X, Banta JT, Ke B, Jiang H, He J, Liu C, Wang J. Wavefront derived refraction and full eye biometry in pseudophakic eyes. PLoS One 2016;11(3):e0152293.
13 Asiedu K, Kyei S, Ampiah EE. Autorefraction, retinoscopy, Javal’s rule, and Grosvenor’s modified Javal’s rule: the best predictor of refractive astigmatism. J Ophthalmol 2016;2016:3584137.
14 de Juan V, Herreras JM, Martin R, Morejon A, Perez I, Rio-Cristobal A, Rodriguez G. Repeatability and agreement of ARK-30 autorefraction after cataract surgery. Clin Exp Ophthalmol 2012;40(2):134-140.
15 Venter J, Pelouskova M. Outcomes and complications of a multifocal toric intraocular lens with a surface-embedded near section. J Cataract Refract Surg 2013;39(6):859-866.
16 Muñoz G, Albarrán -Diego C, Ferrer-Blasco T, Sakla HF, García-Lázaro S. Visual function after bilateral implantation of a new zonal refractive aspheric multifocal intraocular lens. J Cataract Refract Surg 2011;37(11):2043-2052.
17 Muñoz G, Albarrán -Diego C, Javaloy J, Sakla HF, Cerviño A.Combining zonal refractive and diffractive aspheric multifocal intraocular lenses. J Refract Surg 2012;28(3):174-181.
18 Chiam PJ, Quah SA. The refractive outcome of Toric Lentis Mplus implant in cataract surgery. Int J Ophthalmol 2016;9(5):699-702.
19 Jorge J, Queirós A, Almeida JB, Parafita MA. Retinoscopy/autorefraction:which is the best starting point for a noncycloplegic refraction? Optom Vis Sci 2005;82(1):64-68.
20 McNeely RN, Pazo E, Spence A, Richoz O, Nesbit MA, Moore TC,Moore JE. Comparison of the visual performance and quality of vision with combined symmetrical inferonasal near addition versus inferonasal and superotemporal placement of rotationally asymmetric refractive multifocal intraocular lenses. J Cataract Refract Surg 2016;42(12):1721-1729.