INTRODUCTION
Pituitary adenomas, especially nonfunctioning pituitary adenomas, have many opportunities to produce visual impairment due to damage to the optic chiasm, including direct compression and disturbances of the blood supply of the optic chiasm[1-6]. Visual dysfunction can be associated with visual acuity (VA) deterioration, visual field (VF) defects, color vision deficit, optic disc atrophy and oculomotor abnormalities[7-8].
Surgery can improve vision in most patients with pituitary adenoma by excision of the lesions decompressing the anterior visual pathways[7,9-10]. Because visual recovery situations vary among patients, preoperative evaluation and predictive prognosis are becoming common issues of concern for both neurosurgeons and patients.
Some studies have focused on the prediction of visual recovery after pituitary adenoma surgery. By using statistical analysis methods, these studies have determined the relationships between all possible factors and visual function before and after treatment, as well as the best prognostic factors. With our growing understanding of the close relationships among changes in the retina, the deterioration of visual function and damage to the optic chiasm induced by pituitary adenoma,the retina has gradually become the focus of research for the postoperative visual recovery prediction of patients with pituitary adenoma. Besides the characteristics of the patients and tumors, retinal layer measurements, especially retinal nerve fiber layer (RNFL) thickness measured by optical coherence tomography (OCT) devices[11-13], have been used to reveal the relationship between changes in the pituitary gland and visual function loss.
Individual studies have revealed the links between pituitary adenoma and the optic chiasm or the retina, but there has been a lack of summarization of the results from studies of the predictive factors for visual recovery after pituitary adenoma resection. This study fills this gap by reviewing related articles,particularly determining the dominant predictive factors in predicting the postoperative visual outcome of patients with pituitary adenoma.
MATERIALS AND METHODS
Literature Search Strategy The literature review considered studies of the postoperative visual recovery prediction of patients with pituitary adenoma. In the following databases,data source articles from January 2000 to May 2017 were searched: PubMed, Google Scholar, Web of Science and Cochrane Library. A full-text search was conducted using the following terms: “pituitary adenoma” AND “visual recovery”AND “predictive factors” or “prediction”, AND “retinal thickness” OR “optical coherence tomography” OR “OCT”.No restriction was placed on the language of the publication.The reference sections of the relevant reviews and original articles were also scanned for potential trials that may have been missed in the primary searches.
Inclusion and Exclusion Criteria To reveal the predictive factors in the postoperative visual recovery for pituitary adenoma, the studies included in the Meta-analysis had to meet the following criteria: 1) non-randomized controlled trials (non-RCT), which studied the relationships between the possible predictive factors and the visual function before and after treatment; 2) at least one of the primary outcomes [VF,VA, best-corrected visual acuity (BCVA), RNflthickness], or secondary outcomes [macular thickness, ganglion cell complex(GCC) thickness]; 3) enrolled a minimum of 10 eyes. Studies were excluded if they: 1) included patients with diseases other than pituitary adenoma or pituitary tumor that cause chiasmal compression, including craniopharyngioma and suprasellar meningiomas; 2) had no original data (reviews, comments or letters); 3) were not conducted in humans.
Data Extraction and Quality Assessment In each of the included studies for literature review, the characteristic information (type of study, country and year of publication,the number of subjects and studied eyes, age, and gender) and the detailed research strategy information (study apparatus,observation time points, observation factors and conclusive predictive factors) were extracted. For the Meta-analysis,the raw data of the preoperative and postoperative visual function outcomes were carefully collected. Data that could not be obtained were calculated when necessary. All data were extracted from the published studies, and we did not contact the authors for further information. To avoid bias in the data extraction process, all the data and information collection were independently conducted by two individual researchers (Sun M and Chen XJ) following the selection criteria described above.Any discrepancy was resolved by discussion and consensus.The standardized forms of abstraction database were established in Microsoft Excel. We evaluated the quality of the studies included in the Meta-analysis with the Newcastle-Ottawa Scale (NOS)[14] for non-RCTs. The range of NOS is from 1 to 9. A score ≥7 indicates good quality.
Statistical Analysis Using VF defects, the Meta-analysis revealed the relationships between visual function recovery and several factors, such as the age of the patient, mean deviation(MD) value of preoperative VF, the duration of symptoms, and preoperative peripapillary retinal nerve fiber layer (pRNFL)thickness. All statistical analyses in the Meta-analysis were performed using Review Manager Software (version 5.3;Cochrane Collaboration, Oxford, United Kingdom). The weighted mean difference (WMD) and odds ratio (OR) with 95% confidence interval (CI) were calculated for the statistical analyses of continuous and dichotomous outcomes. Statistical heterogeneity was tested using Chi-square test and quantified by I2. If significant heterogeneity (P<0.1 and I2>50%) was detected in the Meta-analysis, the random-effects model was used to pool the measurements; otherwise, a fixed-effects model was used. A P-value less than 0.05 was considered statistically significant.
RESULTS
The literature search yielded 489 references by the search terms. After duplicates were removed, the titles and abstracts of potentially relevant articles were scanned, and 362 studies were excluded. Among the 19 articles that satisfied the inclusion criteria of the literature review, 9 articles were eligible for inclusion in the Meta-analysis. The flowchart in Figure 1 shows the literature search process.
Summary Characteristics of Included Studies The characteristics and detailed research strategies of the nineteen studies[5,7-8,15-30]are listed in Tables 1 and 2. Eighteen included studies were prospective or retrospective cohort studies, and only one was a prospective case-control study. All the included articles had preoperative and postoperative visual function observations,and the follow-up duration ranged from one week to fiveyears. In these studies, researchers have analyzed different perspectives and have used different equipment, including multifarious ophthalmic devices (VF analyzer, Snellen chart, photo negative response, ophthalmoscope and others),magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI)and OCT. The observation factors included various aspects of visual function (VF, VA, photo negative response, color vision), visual pathway indicators (pRNflthickness, macular GCC volume, area or thickness, the status of optic disk pallor,MRI signal intensity of the optic nerve, DTI tensor parameters of the optic radiation, activation of the visual cortex on fMRI),and the baseline characteristics of the participant (age, sex,duration of symptoms) and tumor (type, size, and suprasellar tumor extension). The main conclusive predictive factors were preoperative VF defect, duration of symptoms, age of the patient, tumor size and preoperative pRNflthickness.
Table 1 Characteristics of included studies in the review of predictive factors for visual recovery after pituitary adenoma resection
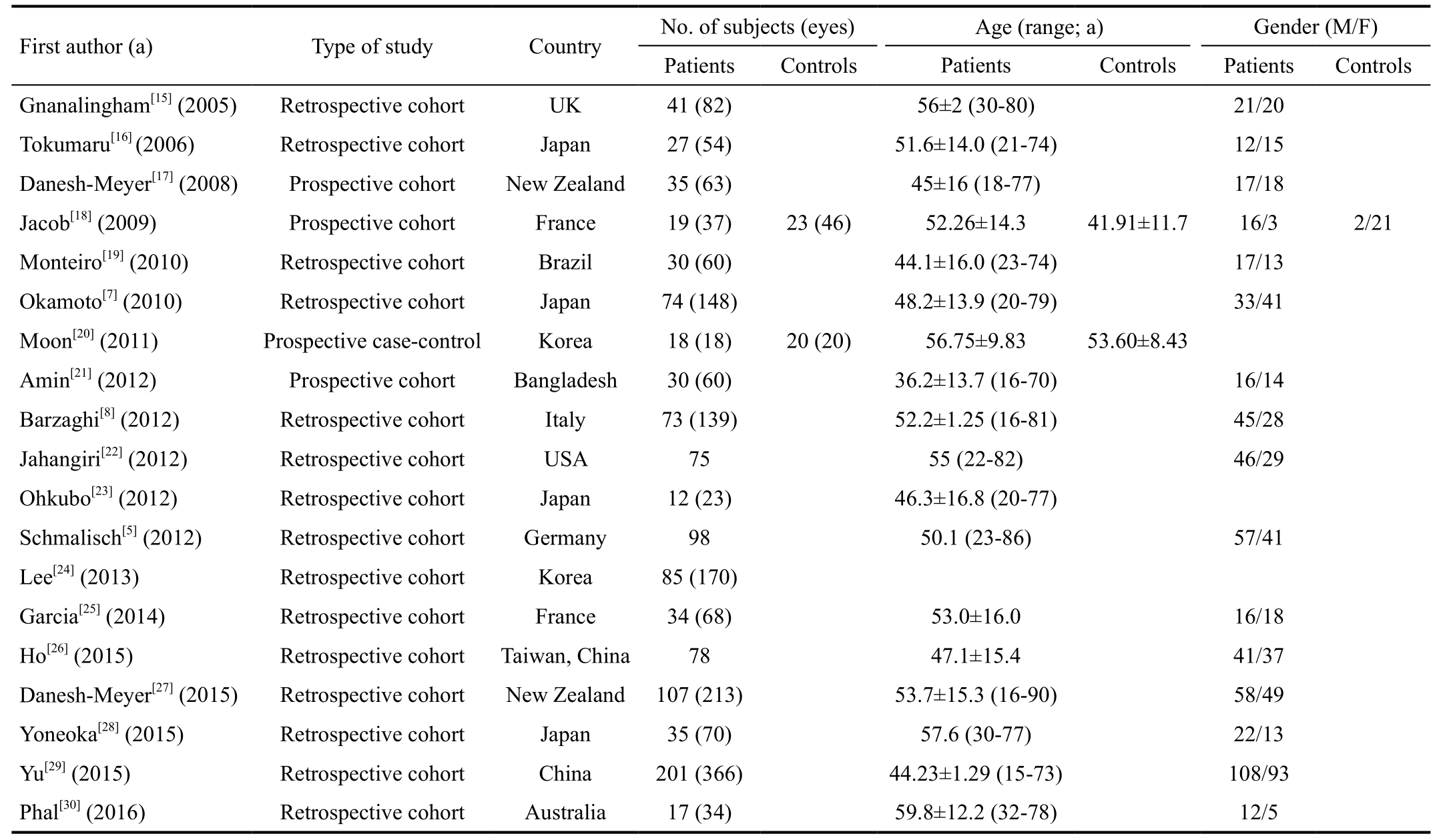
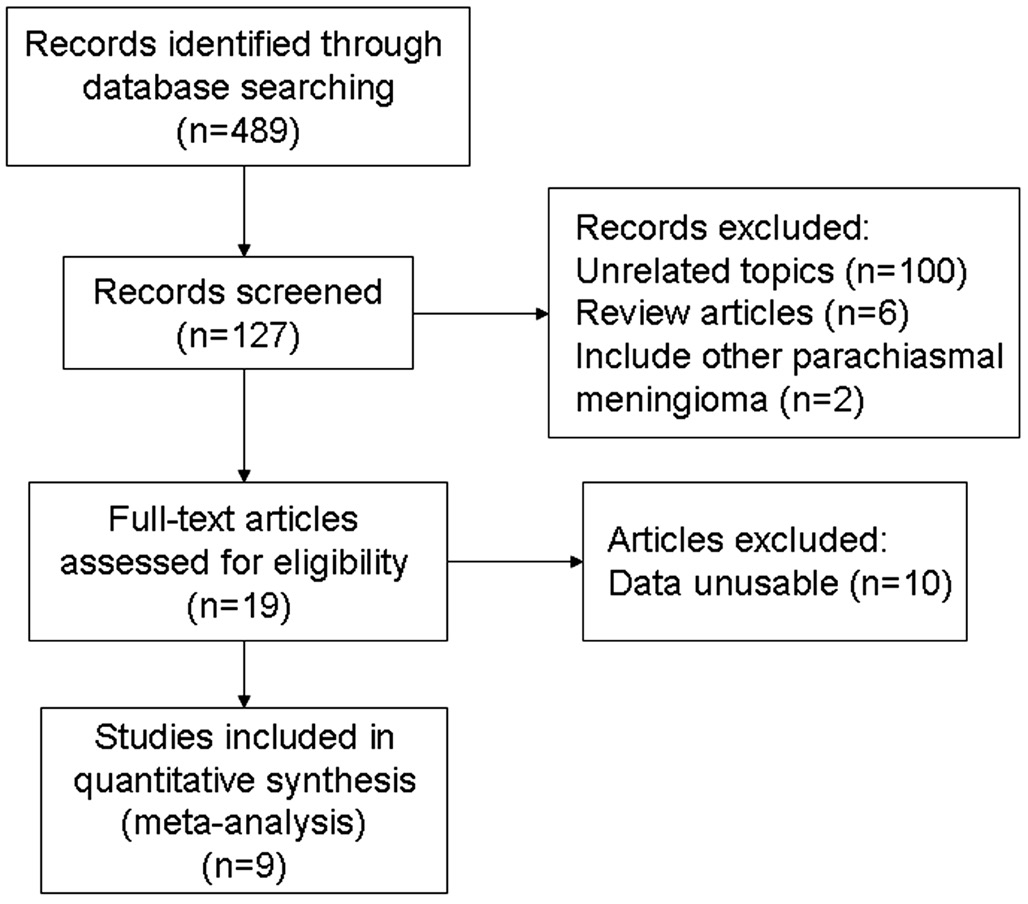
Figure 1 Flow diagram of the literature search in this review.
Visual results from nine studies in the Meta-analysis are listed in Table 3. There were 530 patients, and 975 eyes were analyzed. The sample sizes ranged from 12 to 201. The mean age of the patients in these studies ranged from 36.2 to 59.8y.
Postoperative Visual Field Recovery Preoperative and postoperative VF MD values were available in five trials that included 303 eyes. Five studies showed significant heterogeneity (P=0.0006, I2=80%). A statistically significant difference was found in this outcome (WMD -5.85; 95% CI:-8.19 to -3.51; P<0.00001) (Figure 2).
Preoperative Visual Field Defects and Postoperative Visual Field Recovery Preoperative VF MD values were available in two trials. These studies showed significant heterogeneity(P=0.04, I2=75%). A statistically significant difference was found in this outcome between the sufficient and insufficient postoperative VF improvement groups (WMD 10.09; 95% CI:6.17 to 14.02; P<0.00001), suggesting that less preoperative VF loss could predict better postoperative VF recovery (Figure 3).
Age and Postoperative Visual Field Recovery The preoperative ages of the patients were available in three trials. These studies showed significant heterogeneity (P<0.00001, I2=99%). A significant difference was found in this outcome between thesufficient and insufficient postoperative VF improvement groups (WMD -12.32; 95% CI: -18.42 to -6.22; P<0.0001),suggesting that patients with younger ages could achieve better postoperative VF recovery (Figure 4).
Table 2 Detailed research strategies of included studies
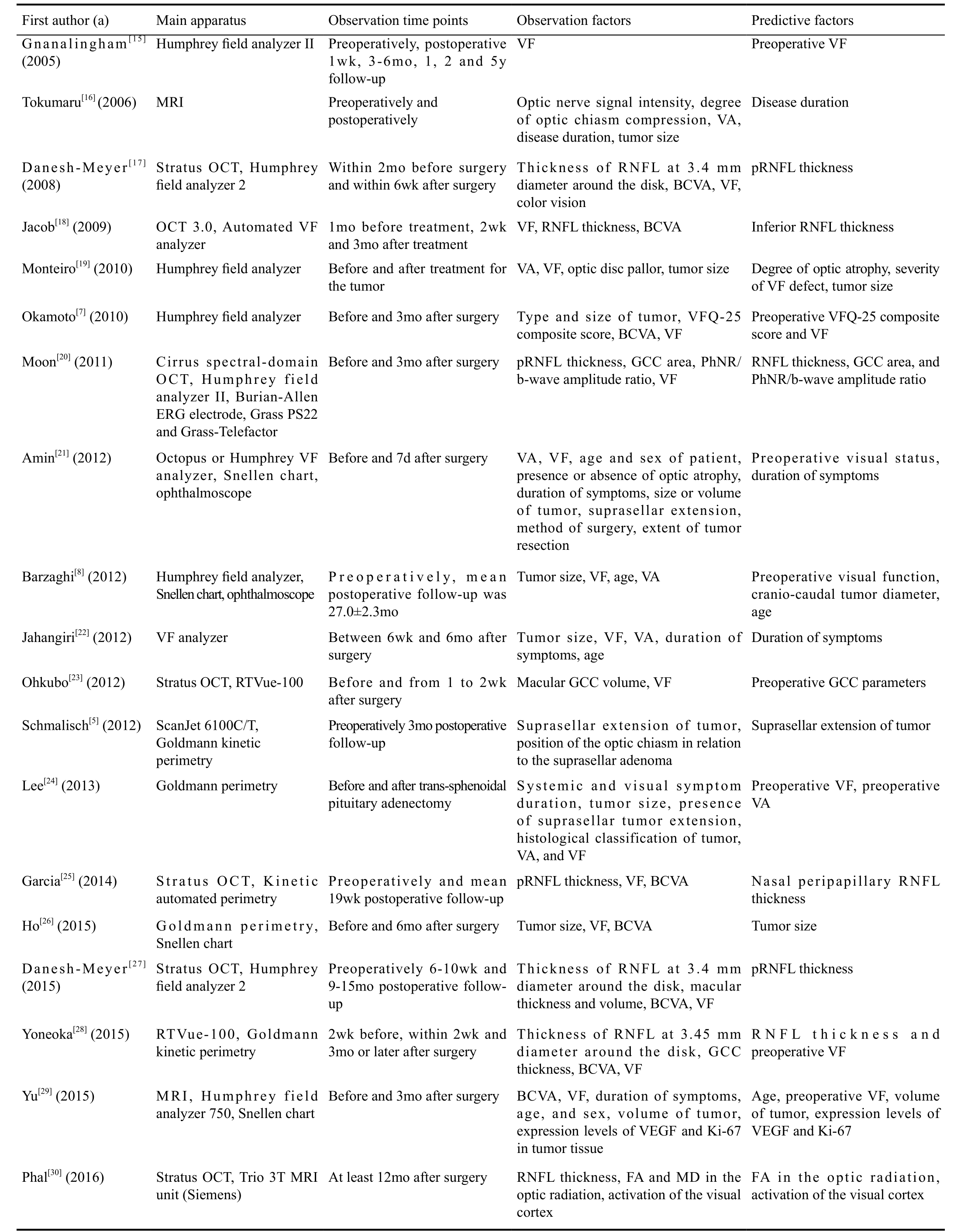
VF: Visual field; MRI: Magnetic resonance imaging; VA: Visual acuity; BCVA: Best-corrected visual acuity; OCT: Optical coherence tomography; RNFL: Retinal nerve fiber layer; pRNFL: Peripapillary retinal nerve fiber layer; VFQ-25: The 25-item National Eye Institute Visual Function Questionnaire; GCC: Ganglion cell complex; PhNR:Photopic negative response; VEGF: Vascular endothelial growth factor; FA: Fractional anisotropy; MD: Mean diffusivity.
Table 3 Visual results from nine studies included in the Meta-analysis
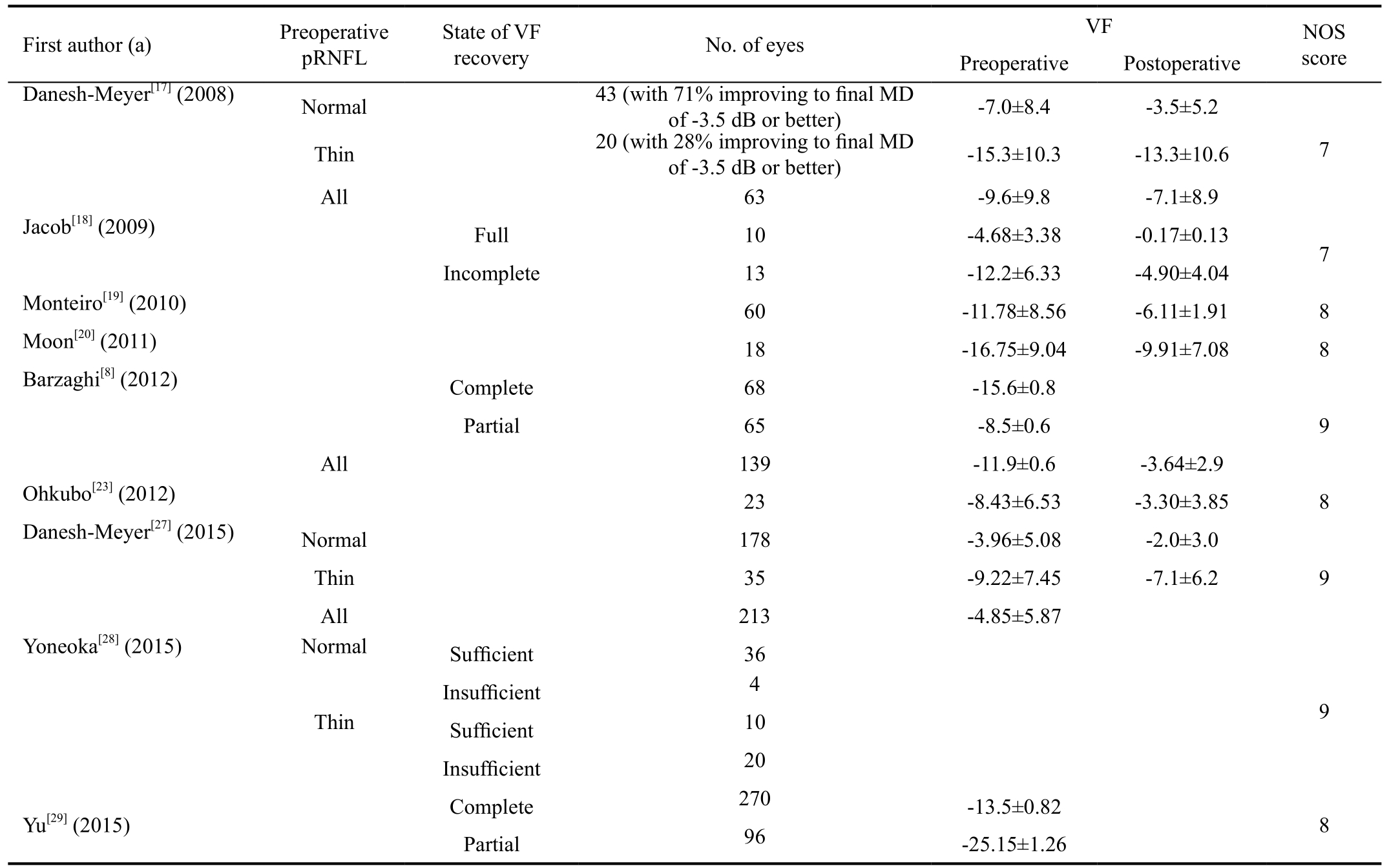
pRNFL: Peripapillary retinal nerve fiber layer; VF: Visual field; MD: Mean diffusivity; NOS: Newcastle-Ottawa Scale.
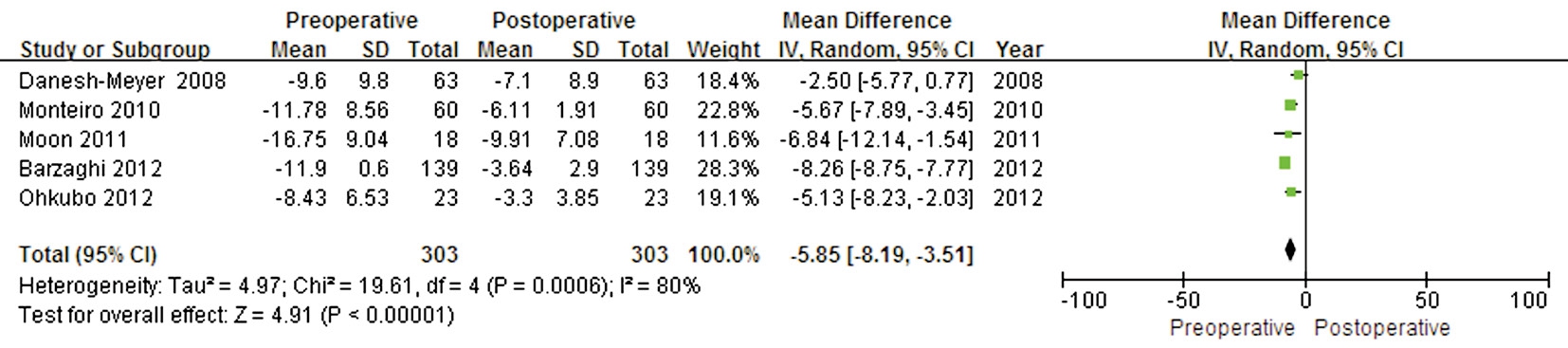
Figure 2 Forest plot of the comparison between the preoperative and postoperative VF values.
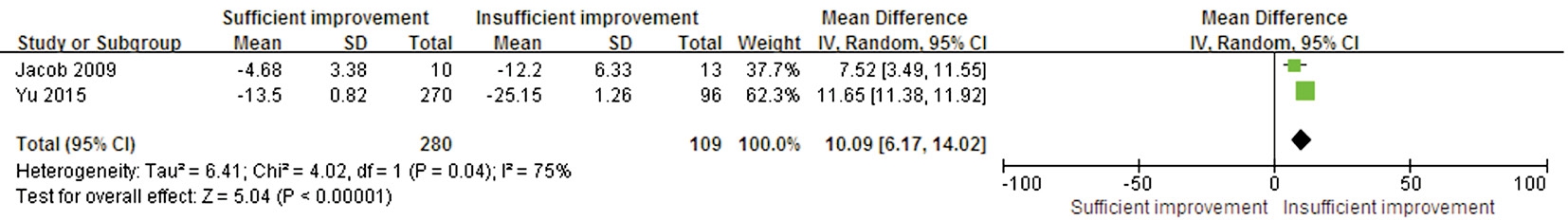
Figure 3 Forest plot of the preoperative VF defect comparison between the sufficient and insufficient postoperative VF improvement groups.
Duration of Symptoms and Postoperative Visual Field Recovery The durations of symptoms were available in three trials. These studies showed significant heterogeneity(P<0.00001, I2=99%). A significant difference was found in this outcome between the sufficient and insufficient postoperative VF improvement groups (WMD -5.04; 95% CI:-9.71 to -0.37; P=0.03), suggesting that a shorter preoperative duration of symptoms could predict better postoperative VF recovery (Figure 5).
Preoperative Peripapillary Retinal Nerve Fiber Layer Thickness and Postoperative Visual Field Recovery A comparison between preoperative normal and thin pRNflin the proportion of eyes with sufficient VF recovery was conducted in two studies. These studies showed moderate heterogeneity (P=0.22,I2=35%); the total effect size OR in these studies was 0.1(95% CI: 0.04 to 0.23), and the Z value was 5.33 (P<0.00001),suggesting that sufficient postoperative VF recovery might be associated with preoperative pRNflthickness (Figure 6).
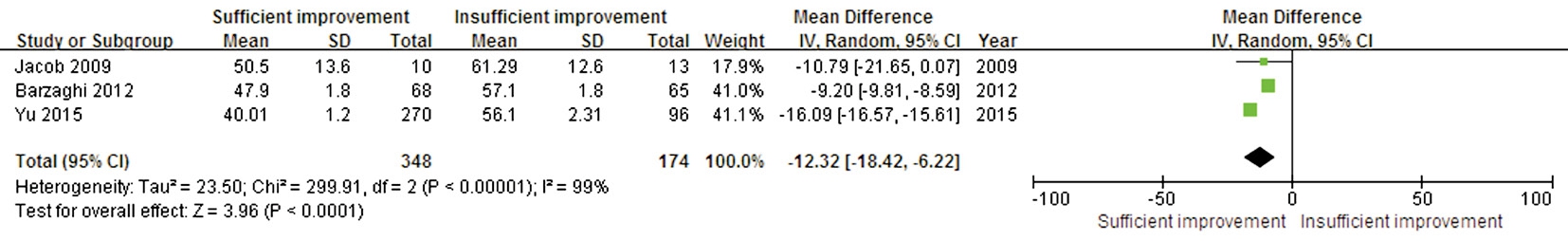
Figure 4 Forest plot of the age comparison between the sufficient and insufficient postoperative VF improvement groups.
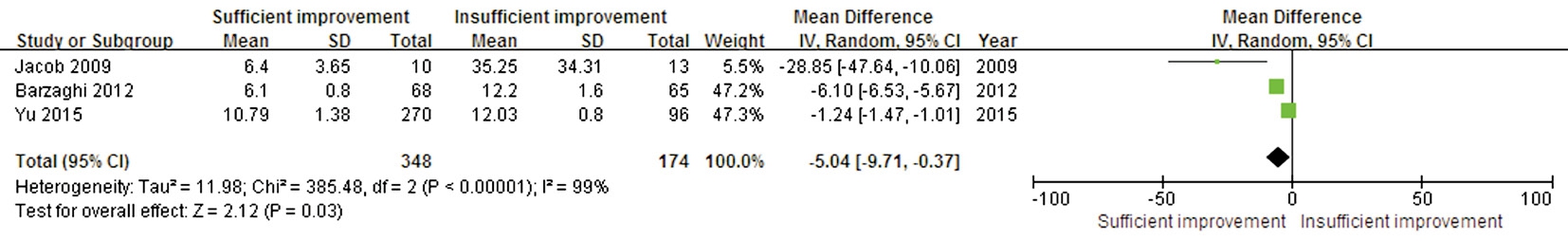
Figure 5 Forest plot of the symptom duration comparison between the sufficient and insufficient postoperative VF improvement groups.
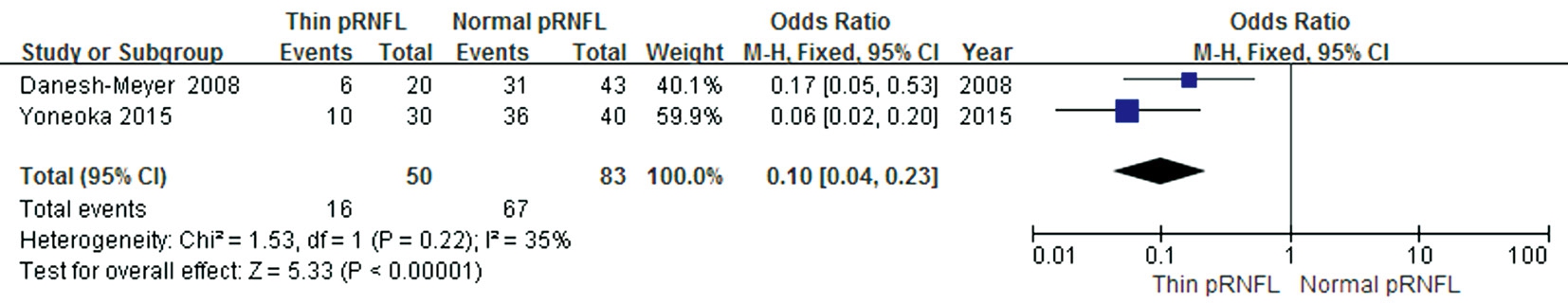
Figure 6 Forest plot of the proportion of eyes with sufficient VF recovery after optic chiasm decompression.
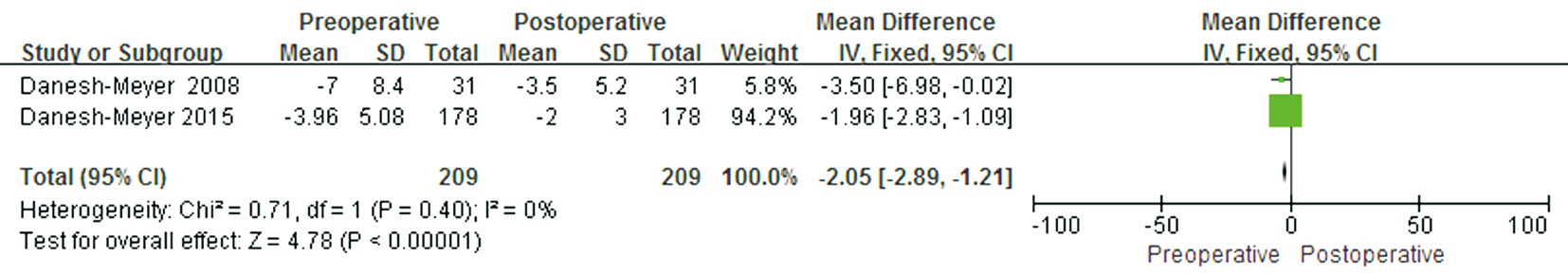
Figure 7 Forest plot of the comparison between the preoperative and postoperative VF for eyes with normal preoperative pRNFL.
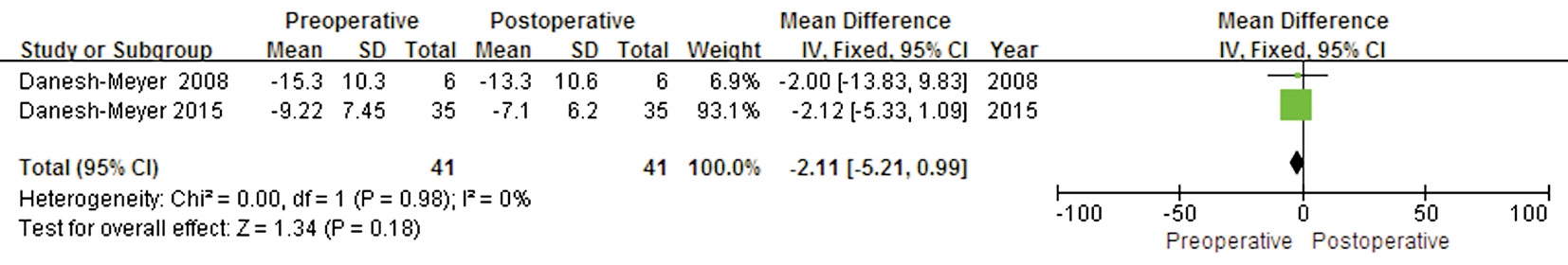
Figure 8 Forest plot of the comparison between the preoperative and postoperative VF for eyes with thin preoperative pRNFL.
A comparison between preoperative and postoperative VF MD values for eyes with normal preoperative pRNflwas conducted in two studies that included 209 eyes. A significant difference was found in this outcome between preoperative and postoperative MD values (WMD -2.05; 95% CI: -2.89 to-1.21; P<0.00001) (Figure 7), suggesting that eyes with normal preoperative pRNflcould achieve good postoperative VF recovery. The heterogeneity test was not significant (P=0.40,I2=0).
A comparison between preoperative and postoperative VF MD values for eyes with thin preoperative pRNflwas conducted in two studies that included 41 eyes. No significant difference was found in this outcome between preoperative and postoperative MD values (WMD -2.11; 95% CI: -5.21 to 0.99; P=0.18), with no evidence of heterogeneity (P=0.98,I2=0) (Figure 8), suggesting that eyes with thin preoperative pRNflthickness could not acquire significant postoperative VF improvement.
DISCUSSION
The studies included in our literature review indicate the dominant predictive factors for the postoperative visual recovery of patients with pituitary adenoma. The Meta-analysis suggests that postoperative VF improvement was significant and factors, such as preoperative VF defects, duration of symptoms, age of the patient, and pRNflthickness measured by OCT, were closely associated with postoperative recovery of VF.
When the optic chiasm is directly compressed, or its blood supply is affected by a pituitary adenoma, retinal ganglion cell(RGC) axonal injury and visual dysfunction will occur. The three main postulated pathological mechanisms are disruption of conduction along the axon, impairment of axoplasmic flow and demyelination with impaired signal conduction[9]. VF defects are the most common and usually the earliest symptom of visual disturbance due to direct compression of the crossing fibers in the optic chiasm by pituitary adenomas. As the disease develops, the macular fibers can be affected and cause other visual dysfunctions, such as VA damage, color vision loss and optic disc pallor. However, VA impairment, color vision loss,and optic disc pallor are strongly associated with the degree of VF defect[8,29]. Therefore, VF defects are emphasized in studies of the correlation between the retina and visual function. The pattern and severity of VF defects depend on the relative position between the optic chiasm and the tumor, as well as the growth direction and size of the tumor[26,31]. Larger tumors with greater upward growth increase the pressure on the chiasm,resulting in a more severe degree of visual loss[19]. However,our Meta-analysis did not include tumor characteristics because the raw data from the included studies consisted of various forms, including volume, size acquired from coronal or sagittal MRI images, which preventing pooling of the data.Surgical removal is a common therapy for pituitary adenoma and can generally improve the visual function of patients with visual symptoms complaints[5,10] (Figure 2). Compared with patients with good preoperative MD values, patients with poor preoperative MD values had worse VF recovery(Figure 3). The duration of visual symptoms refers to the time from the onset of VF loss or diminished VA until the diagnosis of pituitary adenoma. An extended duration of visual symptoms can cause decreased improvement after resection (Figure 5). Excluding subjective delays by patients,visual symptom duration was correlated with the age of the patient. Due to misunderstandings of declining vision among the elderly, older patients are more prone to prolonged visual symptoms than younger patients[22]. Compared with a younger patient, an older patient has a lower probability of recovery(Figure 4). In fact, the total number of neurons is smaller in the older retina than that in the younger retina, and neuronal density is lower in most regions of the older retina[32-33], which might be the pathomechanism underlying this phenomenon.However, if the duration of symptoms is less than 6mo, there is no significant difference in visual improvement between older and younger patients[22].
Although these studies reveal strong correlations between various factors and the prediction of visual recovery after pituitary adenoma surgery, the retina as a determining factor of visual function should be considered. The physiological relationship between the retina and optic chiasm ensures that damage to the optic chiasm will affect the retina, especially the RNFL, which comprises RGC axons. Optic nerve fibers originating from the RGCs through the optic disk form the optic nerves, and they also constitute the optic chiasm, with the nasal hemiretinal fibers decussating, while the temporal fibers uncross after entering the cranium[34]. Axonal injury-induced RGC death and axon loss will cause RNflthinning correlated with visual disturbances. The cutoff values differentiating thin RNflthicknesses from normal thicknesses have usually been defined as 95% or 97.5% of the normal values derived from age-matched normative databases[17-18]. Eyes in the thin nerve group had average preoperative RNflthicknesses less than the cutoff values.
As a prespecified marker of axonal loss, pretreatment pRNflthickness measurements by OCT can present the pattern of axonal loss, which might indicate chiasmal compression[35],and can be used to predict visual outcomes after treatment for pituitary adenoma[36]. Eyes with visual defects but normal preoperative RNflthickness showed a significantly greater improvement in postoperative visual function than those with thin preoperative RNflthickness (Figure 6). Compared with eyes with thin pRNFL, eyes with normal pRNflhad a greater likelihood of achieving approximately normal VFs, indicating an increased propensity for visual recovery (Figures 7 and 8).In addition, if preoperative pRNflthickness was less than a specific cutoff value for thickness, e.g. 85 μm[17], the eyes with thicker pRNfldemonstrated faster restoration of VF defects than those with thinner pRNFL. Long-term follow-up revealed that eyes with normal pRNflshowed the greatest visual recovery within the first 6-10wk after surgery[17,27], and eyes with thin pRNflshowed distinct improvement in the period of 1 to 2y postoperatively[27].
RNflthinning indicates the loss of ganglion cell axons due to long-term chiasmal lesions. Typically, compression of the optic chiasm will induce an immediate mechanical conduction block along the axon, and persistent pituitary adenoma will affect the axoplasmic flow that provides energy to the RGCs. Then, the anterograde (from the retina to the brain) and retrograde (from the brain to the retina) electrical activity will be impaired, and demyelination and RGC loss,known as retrograde degeneration, will occur[2,9,37-39], resulting in psychophysical visual dysfunctions. Such changes in the axons and RGCs reflect the degree of visual impairment due to a pituitary adenoma, although the retina might manifest normal RNflthickness. Eyes with visual dysfunction but normal preoperative RNflthickness had damaged axonal and RGC function accompanied by mostly intact structure,whereas eyes with thin RNflthickness not only had severe visual defects but also had axonal atrophy and RGC death.When damage to the optic chiasm ended after surgery, most of the dysfunctional RGCs recovered activity in eyes with normal preoperative RNFL. Although there was perhaps prolonged retrograde degeneration, axoplasmic flow was restored, and remyelination occurred. For eyes with thin preoperative RNflthickness, the severely affected optic nerve and retina might result in prolonged degeneration and delayed restoration of retinal structure[37], which might be explained by the axonal remyelination that creates new concentric lamellar internodes provided by viable adult oligodendrocytes in close proximity[9,40]. Other possible explanations include remodeling by oligodendrocyte progenitors within the anterior visual pathway[9,41] or re-establishment of the vascular supply that was impeded tumor-induced stretching of the chiasmal blood supply[37].
In conclusion, we presented an overview of studies (published to date) of the predictive factors for visual function recovery after pituitary adenoma resection; the predictive factors generally included preoperative VF, duration of symptoms,age, and pRNflthickness. There were relationships among these factors, and the visual dysfunction induced by pituitary adenoma was ultimately attributed to retinal damage.
ACKNOWLEDGEMENTS
Foundations: Supported in part by the National Basic Research Program of China (973 Program) (No.2014CB748600); the National Natural Science Foundation of China (No.81371629;No.81401472; No.61401293; No.61401294; No.61622114);and the Natural Science Foundation of the Jiangsu Province(No.BK20140052).
Conflicts of Interest: Sun M, None; Zhang ZQ, None; Ma CY, None; Chen SH, None; Chen XJ, None.
REFERENCES
1 Mcllwaine GG, Carrim ZI, Lueck CJ, Chrisp TM. A mechanical theory to account for bitemporal hemianopia from chiasmal compression. J Neuroophthalmol 2005;25(1):40-43.
2 Ventura LM, Venzara FX, Porciatti V. Reversible dysfunction of retinal ganglion cells in non-secreting pituitary tumors. Doc Ophthalmol 2009;118(2):155-162.
3 Bergland R. The arterial supply of the human optic chiasm. J Neurosurg 1969;31(3):327-334.
4 Gould TJ, Johnson LN, Colapinto EV, Spollen LE, Rodriguez FJ.Intrasellar vascular malformation mimicking a pituitary macroadenoma. J Neuroophthalmol 1996;16(3):199-203.
5 Schmalisch K, Milian M, Schimitzek T, Lagreze WA, Honegger J. Predictors for visual dysfunction in nonfunctioning pituitary adenomas-implications for neurosurgical management. Clin Endocrinol 2012;77(5):728-734.
6 Mayson SE, Snyder PJ. Silent (clinically nonfunctioning) pituitary adenomas. J Neurooncol 2014;117(3):429-436.
7 Okamoto Y, Okamoto F, Yamada S, Honda M, Hiraoka T, Oshika T.Vision-related quality of life after transsphenoidal surgery for pituitary adenoma. Invest Ophthalmol Vis Sci 2010;51(7):3405-3410.
8 Barzaghi LR, Medone M, Losa M, Bianchi S, Giovanelli M, Mortini P. Prognostic factors of visual field improvement after trans-sphenoidal approach for pituitary macroadenomas: review of the literature and analysis by quantitative method. Neurosurg Rev 2012;35(3):369-379.
9 Kerrison JB, Lynn MJ, Baer CA, Newman SA, Biousse V, Newman NJ.Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol 2000;130(6):813-820.
10 Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery 2005;56(6):1222-1233.
11 Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Fujimoto JG. Optical coherence tomography. Science 1991;254(5035):1178-1181.
12 Garcia-Martin E, Pinilla I, Idoipe M, Fuertes I, Pueyo V. Intra and interoperator reproducibility of retinal nerve fibre and macular thickness measurements using Cirrus Fourier-domain OCT. Acta Ophthalmol 2011;89(1):e23-e29.
13 Menke MN, Knecht P, Sturm V, Dabov S, Funk J. Reproducibility of nerve fiber layer thickness measurements using 3D Fourier-domain OCT.Invest Ophthalmol Vis Sci 2008;49(12):5386-5391.
14 Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25(9):603-605.
15 Gnanalingham KK, Bhattacharjee S, Pennington R, Ng J, Mendoza N.The time course of visual field recovery following transphenoidal surgery for pituitary adenomas: predictive factors for a good outcome. J Neurol Neurosurg Psychiatry 2005;76(3):415-419.
16 Tokumaru AM, Sakata I, Terada H, Kosuda S, Nawashiro H, Yoshii M.Optic nerve hyperintensity on T2-weighted images among patients with pituitary macroadenoma: correlation with visual impairment. AJNR Am J Neuroradiol 2006;27(2):250-254.
17 Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 2008;49(5):1879-1885.
18 Jacob M, Raverot G, Jouanneau E, Borson-Chazot F, Perrin G,Rabilloud M, Tilikete C, Bernard M, Vighetto A. Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am J Ophthalmol 2009;147(1):64-70.
19 Monteiro MLR, Zambon BK, Cunha LP. Predictive factors for the development of visual loss in patients with pituitary macroadenomas and for visual recovery after optic pathway decompression. Can J Ophthalmol 2010;45(4):404-408.
20 Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 2011;52(11):8527-8533.
21 Amin MR, Nath HD, Hossain MA, Barua KK. Early post-operative visual outcome in patient with pituitary adenoma. Banglad J Neurosci 2012;28(2):108-115.
22 Jahangiri A, Lamborn KR, Blevins L, Kunwar S, Aghi MK. Factors associated with delay to pituitary adenoma diagnosis in patients with visual loss. J Neurosurg 2012;116(2):283-289.
23 Ohkubo S, Higashide T, Takeda H, Murotani E, Hayashi Y, Sugiyama K. Relationship between macular ganglion cell complex parameters and visual field parameters after tumor resection in chiasmal compression. Jpn J Ophthalmol 2012;56(1):68-75.
24 Lee S, Kim SJ, Yu YS, Kim YH, Paek SH, Kim DG, Jung HW.Prognostic factors for visual recovery after transsphenoidal pituitary adenectomy. Br J Neurosurg 2013;27(4):425-429.
25 Garcia T, Sanchez S, Litre CF, Radoi C, Delemer B, Rousseaux P,Ducasse A, Arndt C. Prognostic value of retinal nerve fiber layer thickness for postoperative peripheral visual field recovery in optic chiasm compression. J Neurosurg 2014;121(1):165-169.
26 Ho RW, Huang HM, Ho JT. The influence of pituitary adenoma size on vision and visual outcomes after trans-sphenoidal adenectomy: a report of 78 cases. J Korean Neurosurg Soc 2015;57(1):23-31.
27 Danesh-Meyer HV, Wong A, Papchenko T, Matheos K, Stylli S,Nichols A, Frampton C, Daniell M, Savino PJ, Kaye AH. Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci 2015;22(7):1098-1104.
28 Yoneoka Y, Hatase T, Watanabe N, Jinguji S, Okada M, Takagi M,Fujii Y. Early morphological recovery of the optic chiasm is associated with excellent visual outcome in patients with compressive chiasmal syndrome caused by pituitary tumors. Neurol Res 2015;37(1):1-8.
29 Yu FF, Chen LL, Su YH, Huo LH, Lin XX, Liao RD. Factors influencing improvement of visual field after trans-sphenoidal resection of pituitary macroadenomas: a retrospective cohort study. Int J Ophthalmol 2015;8(6):1224-1228.
30 Phal PM, Steward C, Nichols AD, Kokkinos C, Desmond PM, Danesh-Meyer H, Sufaro YZ, Kaye AH, Moffat BA. Assessment of optic pathway structure and function in patients with compression of the optic chiasm: A correlation with optical coherence tomography MRI assessment of optic chiasm compression. Invest Ophthalmol Vis Sci 2016;57(8):3884-3890.
31 Ogra S, Nichols AD, Stylli S, Kaye AH, Savino PJ, Danesh-Meyer HV.Visual acuity and pattern of visual field loss at presentation in pituitary adenoma. J Clin Neurosci 2014;21(5):735-740.
32 Harman A, Abrahams B, Moore S, Hoskins R. Neuronal density in the human retinal ganglion cell layer from 16-77 years. Anat Rec 2000;260(2):124-131.
33 Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol 2003;87(7):899-901.
34 Jain NS, Jain SV, Wang X, Neely AJ, Tahtali M, Jain S, Lueck CJ.Visualization of nerve fiber orientations in the human optic chiasm using photomicrographic image analysis. Invest Ophthalmol Vis Sci 2015;56(11):6734-6739.
35 Moon CH, Lee SH, Kim BT, Hwang SC, Ohn YH, Park TK.Diagnostic ability of retinal nerve fiber layer thickness measurements and neurologic hemifield test to detect chiasmal compression. Invest Ophthalmol Vis Sci 2012;53(9):5410-5415.
36 Savino PJ. Evaluation of the retinal nerve fiber layer: descriptive or predictive? J Neuroophthalmol 2009;29(3):245-249.
37 Danesh-Meyer HV, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y,Vander Hoorn S. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci 2006;47(11):4827-4835.
38 Moon CH, Hwang SC, Ohn YH, Park TK. The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Invest Ophthalmol Vis Sci 2011;52(11):7966-7973.
39 CioffiGA. Ischemic model of optic nerve injury. Trans Am Ophthalmol Soc 2005;103:592-613.
40 Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics 2011;8(1):117-132.
41 Johansson C, Lindblom B. The role of optical coherence tomography in the detection of pituitary adenoma. Acta Ophthalmol 2009;87(7):776-779.