INTRODUCTION
Somatic cells can be reprogrammed to induced pluripotent stem cells (iPSCs), which also can self-renew and differentiate into cells of three germ layers as embryonic stem cells (ESCs), by the related transcriptional factors Oct4, Klf4,Sox2, and cMyc as fi rst reported by Takahashi and Yamanaka[1].Owing to their abilities to replicate unlimitedly and to differentiate into numerous cell types, generation of iPSCs was regarded as one of the most outstanding breakthroughs in the fi eld of stem cell research. During the following years, there have been significant advances in iPSCs generation, such as generated in a broad range of species and from various cell types[2]. These groundbreaking steps have been expected to evolve to novel regenerative cell therapies with the potential to solve many problems surrounding incurable diseases, such as spinal cord injury[3], neurodegenerative disease[4], heart failure[5-6], diabetes[7], and retinal disease[8]. In particular, the clinical application of iPSCs is expected to solve the problems of immune rejection and ethics that are key obstacles in the current clinical use of ESCs.
A large number of the iPSC lines were generated using genetic manipulation, which is a highly efficient and reproducible method. However, small sequences of the DNA are integrated with the genes coding for reprogramming factors frequently in this process, which raises a major safety issue and has hindered the potential clinical applications of iPSCs. Furthermore,these permanent insertions, such as cMyc known as an oncogene in human cells, potentially increase the risk of tumor formation[9-10]. Moreover, the transgenes can potentially disrupt functional genes even if they are silenced or not expressed. To minimize or avoid the side effects resulting from exogenous DNA integration, a number of methods have been developed to produce iPSCs with potentially reduced risks. For example,substitution of the retrovirus vector with adenovirus vector,which integrate into the genome of target cells at extremely low frequencies and will not transfer residual transgenes into the host genomes[11]. Also nonviral reprogramming systems,such as PiggyBac and Sleeping Beauty transposon systems have been adopted[12]. In addition, the Sendai virus, as negative-sense single-stranded RNA does not pose a danger of genome insertion, was also successfully used to generate iPSCs[13-14]. Besides, the direct delivery of synthetic mRNAs of the four transcriptional factors has been shown to successfully reprogram somatic cells to a pluripotent state[15-16]. Smallmolecule drugs have attracted much attention as another way to avoid introducing genetic material. Deng group completed the Chemical iPSCs (CiPSCs) prepared from mouse embryonic fibroblast cells solely using a cocktail of seven smallmolecule compounds[17]. Although these methods signif i cantly reduced genome integration, the reprogramming eff i ciency is influenced at a great extent. Besides, the instability of some nonintegrative systems (such as mRNA delivery) makes the entire reprogramming procedure laborious. Similarly,direct delivery of recombinant proteins was also reported as a successful means of gene introduction for generating iPSCs[18-19]. These protein-based methods are also attractive for clinical application owing to the absence of breaks in existing host genes and the reactivation of transgenes, which is an important safety advantage for human therapy.
The critical step in this method is to produce large quantities of pure and bioactive proteins. Pan et al[20] obtained the recombinant TAT-fused proteins of Sox2, Oct4, Lin28, and Nanog from insect cell in 2014. Unfortunately, most of the recombinant proteins remained in the nuclei of the Sf9 insect cells, and were much difficult to purify. As indicated above,insect cell expression vector system is not ideal for the highvolume production of these proteins. The E. coli expression vector system can produce a high yield of target proteins with low cost and simple purification procedures in a short time.However, the recombinant proteins usually exist in the form of inclusion bodies in commonly-used expression systems such as pET systems, which need laborious denaturation and refolding[18]. The bioactivity of the recombinant proteins may be impaired by the treatments of denaturizing and refolding thus rendered them useless for reprogramming. In present study, we established a new method to improve the solubility of the recombinant proteins and constructed the transactivator transcription (TAT) and the nuclear localization signal (NLS)sequences to the proteins to guarantee the cell-penetrating capacity.
MATERIALS AND METHODS
Materials HEK 293T cells were purchased from Chinese Academy of Typical Culture Collection Cell Bank (Shanghai,China). Fetal Bovine Serum (FBS), Dulbecco's Modified Eagle Medium (DMEM), ampicillin, chloramphenicol, and Alexa488-conjugated secondary antibody were purchased from Invitrogen (Carlsbad, CA, USA). TAT and NLS sequences were synthesized at GenScript (Nanjing, China). Isopropyl-b-D-thiogalactopyranoside (IPTG), coomassie brilliant blue R250,imidazole, and DAPI were purchased from Sigma-Aldrich(Shanghai, China). Endonucleases of SacI and HindIII, Ni-NTA Agarose Resin were purchased form Thermo Scientific(Hudson, NH, USA). Protein marker, BCA Protein Assay kit and enhanced chemiluminescence (ECL) reagent were from HaiGene (Harbin, China). Antibodies against Oct4, Sox2, Klf4,Myc and rabbit secondary antibody were all purchased from Proteintech (Wuhan, China). LAS-4000 CCD camera system was from FUJIFILM, and DMRE fluorescence microscope was from Leica.
Plasmids Construction To generate the recombinant human reprogramming proteins cMyc, Klf4, Oct4, and Sox2, the codon-optimized sequences harboring a short polypeptide sequence derived from the HIV TAT and NLS polypeptide at N-terminal was synthesized and sub-cloned into the pCold-SUMO vector with restriction site of SacI and HindIII. The two domains were linked by a short linker to assure the fl exibility of the protein structure (Figure 1). All the four constructs were verif i ed by sequencing.
Protein Expression The four constructs of pCold-SUMOTAT-NLS-Protein vector were individually transformed into E.coli BL21 (DE3) Chaperone competent cells which can express the chaperone proteins, and the positive clones were selected on Luria-Bertani (LB) agar plate with antibiotics (50 μg/mL ampicillin and 20 μg/mL chloramphenicol). For the expression of recombinant fusion proteins, the selected clones were grown overnight in 5 mL of LB with the antibiotics. The overnight culture broths were then diluted 1:40 in fresh 200 mL of LB broth containing antibiotics and the cells were grown at 37℃until the OD 600 nm reached approximately 0.2-0.3. Then 2 ng/mL of tetracycline and 2 mg/mL of L-arabinose were added into the medium for induction of the chaperone proteins expression, and the cells were incubated at 37℃ for 3-4h with constant shaking. Recombinant proteins were expressed in cells by induction with IPTG at a fi nal concentration of 0.2 mmol/L,and the cells were incubated for 24h at 15℃, 200 r.p.m. The cells were then harvested at 4℃ by centrifugation at 5000 g,and the pellets were resuspended in lysis buffer (20 mmol/L sodium phosphate, pH 7.4, 150 mmol/L NaCl, 8 mmol/L imidazole, and 10% glycerol). After that, the cells were disrupted by sonication on ice at 400 W for 100 cycles, 4s each cycle. Insoluble materials were removed by centrifugation at 8000 g at 4℃ for 15min. To determine the expression and the solubility of target proteins, the supernatants and the total amounts of cell proteins were both analyzed on 12% SDS-polyacrylamide gel and detected by Coomassie brilliant blue R250.

Figure 1 Schematic representation of the four recombinant reprogramming proteins Human reprogramming transcriptional factors were fused to the C-terminus of the 6His and SUMO tags, while the TAT polypeptide and NLS polypeptide were directly fused to the N-terminus of the reprogramming factors. A short linker to assure the fl exibility of the protein structure was inserted between the SUMO tag and TAT sequence.
Protein Purif i cation Totally 20 mL of the supernatants after sonication and centrifugation were collected and added to 1 mL Ni-NTA Agarose Resin pre-equilibrated in 10 mL of lysis buffer (20 mmol/L sodium phosphate, pH 7.4, 150 mmol/L NaCl, 8 mmol/L imidazole, and 10% glycerol) at 4℃. The unbound proteins were removed with wash buffer (20 mmol/L sodium phosphate, pH 7.4, 150 mmol/L NaCl, 20 mmol/L imidazole, and 10% glycerol). Then the four recombinant proteins were eluted with 5 mL of elution buffer (20 mmol/L sodium phosphate, pH 7.4, 150 mmol/L NaCl, 250 mmol/L imidazole, and 10% glycerol). After that, the purif i ed proteins were dialyzed in the storage buffer containing 20 mmol/L sodium phosphate, pH 7.4, 150 mmol/L NaCl, and 10% glycerol at 4℃ for 48h. After purification, the proteins were subjected to 12% SDS-polyacrylamide gel and stained with coomassie brilliant blue R250. The middle-ranged protein marker was used as the protein standard. The concentrations were determined using a commercial BCA Protein Assay Kit and the purif i ed proteins were stored at -80℃.
Western Blot Analysis Proteins were separated on 12% SDS-polyacrylamide gel under denaturing conditions, and then transferred onto a nitrocellulose membrane. Primary antibodies against Oct4, Sox2, Klf4 and cMyc were used to identify the reprogramming proteins correspondently. The membrane was blocked in 5% non-fat milk in 20 mmol/L Tris-buffered saline(pH 7.6) containing 0.05% Tween 20 (TBST) for 1h at room temperature. Then, the membrane was individually incubated with Oct4, Klf4, Sox2, and cMyc primary antibody overnight at 4℃ (1000-folds dilution in TBST buffer containing 5%nonfat powdered milk). And then the membrane was incubated with HRP-conjugated secondary antibody (10 000-folds diluted in TBST buffer containing 5% non-fat powdered milk). The signals were developed using enhanced chemiluminescence reagent and the digital images were captured using a LAS-4000 CCD camera system.
Transduction of Reprogramming Proteins into HEK 293T Cells To test the penetrability of the four recombinant proteins(TAT-NLS-Oct4, TAT-NLS-Klf4, TAT-NLS-Sox2, and TATNLS-cMyc), the HEK 293T cells of 1×104 were seeded in 24-well plates and cultured in DMEM medium with 10%FBS overnight. After washed with PBS twice carefully, cells were cultured for 8h at 37℃ in the new serum-free medium supplemented with recombinant reprogramming proteins (TATNLS-Oct4, TAT-NLS-Klf4, TAT-NLS-Sox2 or TAT-NLS-cMyc) at a fi nal concentration of 8 µg/mL as recommended in reference[17]. After that, cells were incubated for 24h with the replaced complete growth medium.
Immunof l uorescence Analysis HEK 293T cells were washed twice with PBS and then subjected to fi xation. After fi xed in 4% paraformaldehyde for 20min at room temperature, cells were permeabilized with 0.1% Triton X-100 in PBS for 15min at room temperature. The cells were washed three times with PBS and incubated with 5% normal donkey serum for 1h at room temperature. Subsequently, cells were incubated with the primary antibody of Anti-Oct4 (1:200 dilution), Anti-Klf4 (1:200 dilution), Anti-Sox2 (1:400 dilution), and AnticMyc (1:400 dilution) for 2h at room temperature. After washed three times with PBS, cells were incubated with the Alexa488-conjugated secondary antibody (1:200) for 60min at room temperature. Then cells were rinsed with PBS twice for 3min each time. Then, the samples were incubated in the moist chamber for 10min with DAPI (0.5 μg/mL) for nuclear staining. Finally, the cells were washed three times again and were examined by fl uorescence microscope.
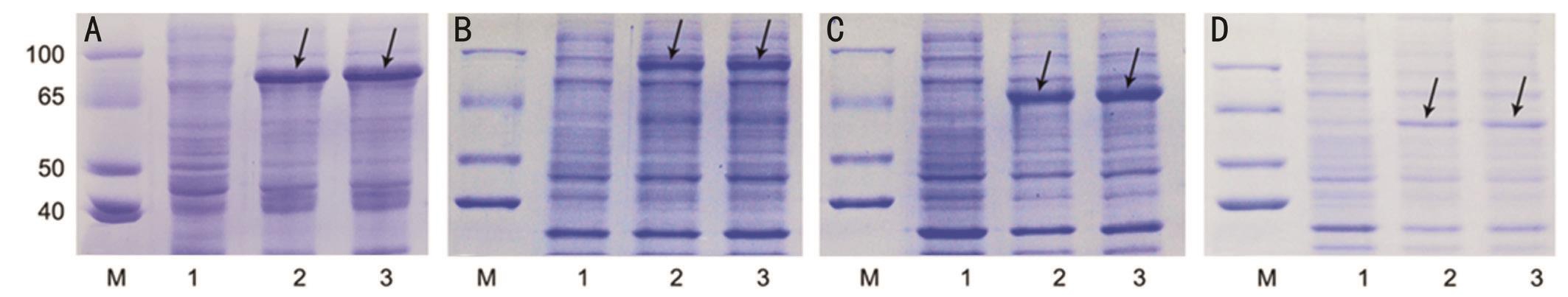
Figure 2 Soluble expression of the four reprogramming factors in E .coli BL21 (chaperone) The four recombinant reprogramming proteins were expressed in E. coli BL21 (Chaperone). The cells lysates with and without IPTG induction and the supernatant after induction were collected for SDS-PAGE and coomassie brilliant blue staining. A: cMyc; B: Klf4; C: Oct4; D: Sox2. Lane M: Protein molecular weight marker;Lane 1: Cell lysates without IPTG induction; Lane 2: Cell lysates after IPTG induction; Lane 3: Supernatant after IPTG induction. The arrows indicate the expected size of the recombinant proteins.
RESULTS
Construction of the SUMO-TAT-NLS Fusion Reprogramming Proteins Expression Vectors It was indicated in previous study that the four reprogramming proteins fused with TAT sequence were commonly expressed in the form of inclusion bodies in E. coli and it was diff i cult to produce high-volume soluble proteins[17]. To obtain soluble proteins, each gene with codon optimized for E. coli was inserted into pCold-SUMO vector. pCold-SUMO expression system was employed to improve the solubility of the proteins by the mechanism of the cold-inducible promoter, SUMO tag and its TEE signal peptide with the assistance of chaperone proteins. To generate recombinant proteins that are able to penetrate the plasma membrane of cells, an 11-amino acid (YGRKKRRQRRR)membrane-penetrating domain derived from the human immunodef i ciency virus type 1 (HIV) Tat protein (TAT) was fused to the N-terminus of the four reprogramming factors individually. Adjacent to the TAT peptides, a 6-amino acid(KKKRKV) nuclear localization signal (NLS) sequence was inserted, which facilitated nuclear localization,potentially decreasing the quantity of proteins trapped in organelles[20-21]. Between the reprogramming proteins and SUMO-TAT-NLS tags, a short linker (GGGGC) was inserted to assure the flexibility of the protein structures (Figure 1).The 6-his tag at N-terminal of the SUMO tag upon the vector was used for purif i cation of the proteins by Ni-aff i nity chromatography.
Soluble Expression of the SUMO-TAT-NLS-cMyc/Klf4/Oct4/Sox2 Fusion Proteins In order to acquire soluble and bioactive proteins, pCold-SUMO expression system was employed. E. coli BL21 (DE3) chaperone, in which chaperone proteins were overexpressed to facilitate protein folding in correct format, was used as the expression host strain. The theoretical molecular mass of cMyc (612aa) , Klf4 (637aa),Oct4 (518aa), and Sox2 (475aa) fusion proteins containing the SUMO-TAT-NLS peptide were approximately 68 kDa, 68 kDa,56 kDa, and 53 kDa respectively. As revealed by SDS-PAGE and Coomassie brilliant blue staining analysis, all the four fusion proteins were overexpressed in total lysates by IPTG induction, and mainly expressed in the soluble fraction (Figure 2).Therefore, it was clearly indicated that our strategy was preferred for soluble expression of the four reprogramming factors.
Purif i cation of the SUMO-TAT-NLS-cMyc/Klf4/Oct4/Sox2 Fusion Proteins Since the reprogramming proteins were co-expressed with an N-terminal his tag in bacteria, the Ni-NTA Resin was applied for purification. As shown in Figure 2, recombinant proteins mainly existed in soluble format in supernatants. The soluble supernatants of each protein were collected and performed for purification with Ni-NTA resin. After washing of the unbound materials by imidazole(20 mmol/L), the proteins were purified successfully with a purity of over 70% (Figure 3A). For further identif i cation, the recombinant proteins were separated on SDS-PAGE followed by Western blot analysis. As shown in Figure 3B, each of the purified protein was recognized by its responding specific primary antibody, showing a single band corresponding to their molecular weight. Therefore, the results indicated that these four reprogramming proteins could be produced in a soluble format through pCold-SUMO expression system and Ni-NTA Resin purif i cation.
Transduction of the SUMO-TAT-NLS-cMyc/Klf4/Oct4/Sox2 Fusion Proteins into Live Cells In our work, the TAT sequence and NLS were fused to the protein constructs. The TAT sequence was designed to facilitate cell penetration of the recombinant proteins, and the NLS sequence was to promote nuclear localization, which is able to potentially decrease the quantity of proteins trapped in organelles. The aim of this study is to obtain the soluble recombinant reprogramming proteins that should also be able to penetrate into the plasma membrane of living cells. In order to verify that, HEK 293T cells as the model were used to determine the penetrating capacity of the proteins. Immunofluorescence staining was performed to confirm the ability of the four recombinant reprogramming proteins to penetrate into HEK 293T cells. Cells were cultivated for 8h individually in medium supplemented with the four reprogramming proteins at a concentration of 8 µg/mL individually, and then incubated in complete medium for 24h. As shown in Figure 4, all the four recombinant proteins could penetrate into nuclei of the cells (shown with green fluorescence signal). Consequently, it was indicated that the four recombinant reprogramming proteins fused with TAT and NLS sequences can penetrate into live cells exactly, which addressed that these proteins obtained through our method would be very useful for iPSCs generation from various other types of cells.
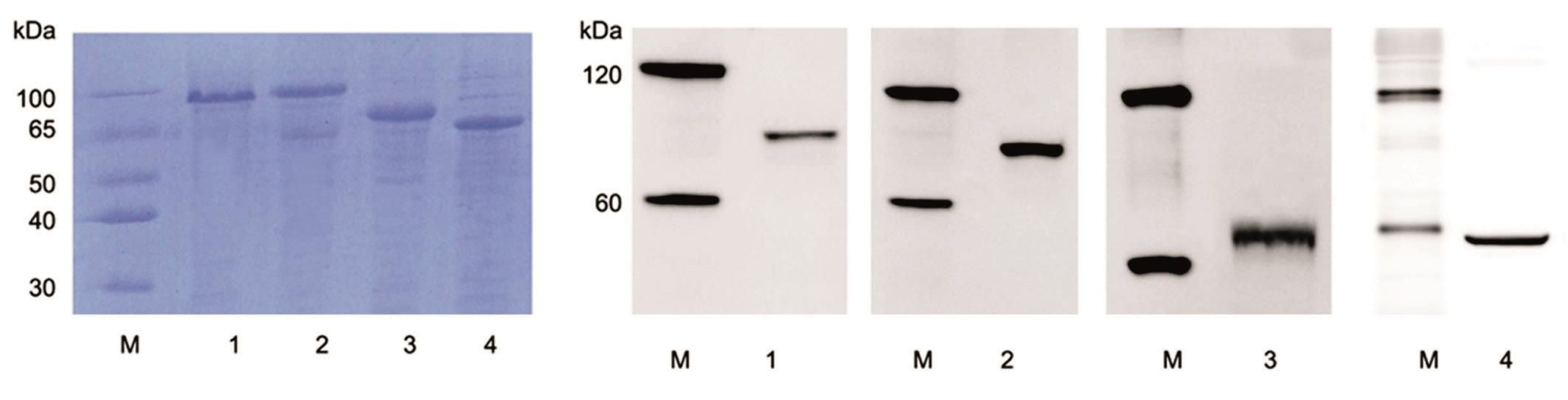
Figure 3 SDS-PAGE and Western blot analysis of the purif i ed recombinant proteins The supernatants of the four proteins were purif i ed by Ni-NTA aff i nity resin, and then were conducted to SDS-PAGE and Western blot analysis. A: SDS-PAGE analysis of the purif i ed recombinant reprogramming proteins by Coomassie blue staining. Lane M: Protein molecular weight marker; Lane 1-4 represented purif i ed cMyc, Klf4, Oct4,and Sox2 proteins respectively. B: Western blot analysis of the each purif i ed protein by specif i c primary antibody against cMyc, Klf4, Oct4, and Sox2 individually. Lane M: Prestained Western blot marker; Lane 1-4 represented purif i ed cMyc, Klf4, Oct4, and Sox2 proteins respectively.
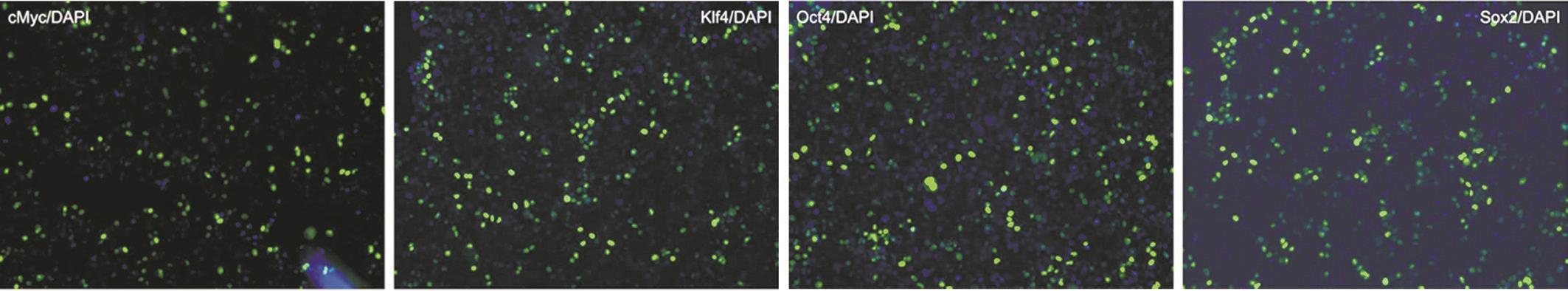
Figure 4 Transduction of TAT-NLS-tagged reprogramming proteins into HEK 293T cells were examined by immunof l uorescence TATNLS-cMyc, TAT-NLS-Klf4, TAT-NLS-Oct4, and TAT-NLS-Sox2 proteins (8 μg/mL) were added to HEK 293T cells. Cells were incubated with the medium supplemented with the corresponding protein for 8h, and then incubated with the complete growth medium for 24h. Cells were fi xed and immunostained with specif i c antibodies and Alexa488-conjuagated secondary antibody (Green). DAPI staining was performed to visualize the nuclei (blue), and the images were merged.
DISCUSSION
In this study, we aimed to prove the feasibility of producing soluble and bioactive reprogramming proteins for iPSCs generation in E. coli, which is the most widely used approach to produce recombinant proteins. Here, the four recombinant proteins were fused to SUMO tag and the E. coli bacteria containing chaperones was employed as host strain, which can extremely decrease the misfolding of these proteins.Subsequently, the four reprogramming proteins were expressed in bacteria and purified from soluble fractions successfully.The fused TAT and NLS sequences facilitate the proteins to penetrate into live cells eff i ciently. Thus the present described method possessed advantages over other non-DNA introducing strategies. First, it can avoid the introduction of exogenous genes absolutely, particularly those that encode intracellular proteins. Second, the recombinant proteins are soluble and bioactive because they have been correctly post-translationally modified and folded. Third, the easily scalable culture is conductive to large-scale production of these recombinant proteins with higher yields. Actually, these purified proteins were quite stable with scarcely precipitation after frozen at-80℃ in 10% glycerol for three months. This characteristic enables the widespread use of our method for generation of iPSCs.
As reported in previous studies, the integration of transgenes into the genome involves genome integration and genetic manipulation, which are complicated by the potential risks,such as insert mutations or tumorigenesis of host genome.Recently, a large number of papers have been published that focused on the safety and efficiency of generation of iPSCs,especially safety[18-19]. Therefore, for the sake of safe clinical application, nonintegrating or non-DNA overexpression strategies for iPSCs generation should be applied[22]. Recently,several approaches have been developed to generate transgene-free or integration-free cell reprogramming[23].Chemical genetics that uses small modulators involved in the regulation of cell states, which is faster, reversible, and more controllable[24]. Another rational approach to achieve nongenetic reprogramming cells is the uses of reprogramming proteins with cell-penetrating peptides (CPPs)[25]. Our recombinant proteins were fused in frame to the TAT peptide sequence derived from the human HIV, and to the NLS sequence, which had a double role of supporting the nuclear localization of the proteins, while minimizing the endosomal/lysosomal trapping and ultimately the degradation of the cargo[21]. Direct delivery of reprogramming proteins to cells was also reported as a good way to induce the generation of iPSCs. The E. coli expression system can produce a high yield of target proteins in a short time with low cost, and the protein purification procedure is simple. Unfortunately, the four recombinant proteins must be released from the inclusion bodies under denaturing conditions in E. coli expression system[19]. Meanwhile, insect cells also can be selected as the expression host. However, none of these recombinant proteins were expressed as secreted proteins, and all of them were detected only in the cell debris. This characteristic of these proteins made purif i cation diff i cult and limited their utility[20].Although the direct transduction of reprogramming proteins to live cells has been proved to be a successful method to induce iPSCs, the reprogramming efficiency remains to be an issue that deserves consideration. The reprogramming eff i ciency of purif i ed protein transduction appears to be lower than that of gene introduction, which maybe caused by the cellular uptake mechanisms[21]. The combinative uses of small molecule VPA regimen and recombinant proteins with CPPs showed significantly higher reprogramming efficiency than their separate application[18,26]. The efficacy of reprogramming is also inf l uenced by the protein concentration and the frequency of the treatment[26]. In several reprogramming systems,improvement was achieved by the addition of certain small molecules[27-28]. In view of these fi ndings, further optimization of the process to increase the reprogramming rate will be investigated in our further study.
ACKNOWLEDGEMENTS
Foundation: Supported by the Foundation of Heilongjiang Provincial Foundation for Youths Project (No.QC2011C119).
Conflicts of Interest: Liu GD, None; Zhou SF, None; Ding XC, None; Fang CL, None; Mi SY, None; Gao XC, None;Han Q, None.
REFERENCES
1 Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126(4):663-676.
2 Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fi broblasts by def i ned factors. Cell 2007;131(5):861-872.
3 Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res 2013;23(1):70-80.
4 Ross CA, Akimov SS. Human-induced pluripotent stem cells: potential for neurodegenerative diseases. Hum Mol Genet 2014;23(R1):R17-R26.5 Fujita J, Itabashi Y, Seki T, Tohyama S, Tamura Y, Sano M, Fukuda K.Myocardial cell sheet therapy and cardiac function. Am J Ρhysiol Heart Circ Ρhysiol 2012;303(10):H1169-H1182.
6 Hsiao LC, Carr C, Chang KC, Lin SZ, Clarke K. Stem cell-based therapy for ischemic heart disease. Cell Transplant 2013;22(4):663-675.
7 Holditch SJ, Terzic A, Ikeda Y. Concise review: pluripotent stem cellbased regenerative applications for failing β-cell function. Stem Cells Transl Med 2014;3(5):653-661.
8 Ramsden CM, Powner MB, Carr AJ, Smart MJ, da Cruz L, Coffey PJ.Stem cells in retinal regeneration: past, present and future. Development 2013;140(12):2576-2585.
9 Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007;448(7151):313-317.
10 Jalving M, Schepers H. Induced pluripotent stem cells: will they be safe? Curr Opin Mol Ther 2009;11(4):383-393.
11 Harui A, Suzuki S, Kochanek S, Mitani K. Frequency and stability of chromosomal integration of adenovirus vectors. J Virol 1999;73(7):6141-6146.
12 Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M,Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fi broblasts to induced pluripotent stem cells. Nature 2009;458(7239):766-770.
13 Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Ρroc Jpn Acad Ser B Ρhys Biol Sci 2009;85(8):348-362.
14 Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H,Tohyama S, Hashimoto H, Kodaira M, Okada Y, Seimiya H, Fusaki N,Hasegawa M, Fukuda K. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010;7(1):11-14.
15 Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W,Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ,Cowan C, Schlaeger TM, Rossi DJ. Highly eff i cient reprogramming to pluripotency and directed differentiation of human cells with synthetic modif i ed mRNA. Cell Stem Cell 2010;7(5):618-630.
16 Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y,Zhang Y, Yang W, Gruber PJ, Epstein JA, Morrisey EE. Highly eff i cient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 2011;8(4):376-388.
17 Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W,Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013;341(6146):651-654.
18 Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G,Yao S, Zhu Y, Siuzdak G, Schöler HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009;4(5):381-384.
19 Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009;4(6):472-476.
20 Pan C, Jia W, Lu B, Bishop CE. Expression of TAT recombinant Oct4,Sox2, Lin28, and Nanog proteins from baculovirus-infected Sf9 insect cells. Gene 2015;556(2):245-248.
21 Nemes C, Varga E, Polgar Z, Klincumhom N, Pirity MK, Dinnyes A. Generation of mouse induced pluripotent stem cells by protein transduction. Tissue Eng Ρart C Methods 2014;20(5):383-392.
22 Inagawa K, Ieda M. Direct reprogramming of mouse fi broblasts into cardiac myocytes. J Cardiovasc Transl Res 2013;6(1):37-45.
23 Zhou YY, Zeng F. Integration-free methods for generating induced pluripotent stem cells. Genomics Ρroteomics Bioinformatics 2013;11(5):284-287.
24 Alexanian AR, Liu QS, Zhang Z. Enhancing the eff i ciency of direct reprogramming of human mesenchymal stem cells into mature neuronallike cells with the combination of small molecule modulators of chromatin modifying enzymes, SMAD signaling and cyclic adenosine monophosphate levels. Int J Biochem Cell Biol 2013;45(8):1633-1638.
25 Thier M, Münst B, Edenhofer F. Exploring refined conditions for reprogramming cells by recombinant Oct4 protein. Int J Dev Biol 2010;54(11-12):1713-1721.
26 Zhang H, Ma Y, Gu J, Liao B, Li J, Wong J, Jin Y. Reprogramming of somatic cells via TAT-mediated protein transduction of recombinant factors. Biomaterials 2012;33(20):5047-5055.
27 Mali P, Chou BK, Yen J, Ye Z, Zou J, Dowey S, Brodsky RA, Ohm JE, Yu W, Baylin SB, Yusa K, Bradley A, Meyers DJ, Mukherjee C,Cole PA, Cheng L. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 2010;28(4):713-720.
28 Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fi broblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 2008;3(5):568-574.