INTRODUCTION
Cataract and glaucoma are the main leading causes of blindness in the world[1]. In the aging population, they frequently occur together. Clear corneal phacoemulsification(CCP) is associated with signif i cant and sustained reduction in intraocular pressure (IOP) in both normal subjects and patients with primary open angle glaucoma (POAG)[2-6]. Several hypotheses have been proposed explaining the mechanism of IOP decrease. One of the more supported theories describes the increased outf l ow of aqueous humor by endogenous secretion of prostaglandins and interleukins[7].
Selective laser trabeculoplasty (SLT) is a relatively new technique to lower IOP. Most studies on the effectiveness of SLT compare rather small populations and lack power.However, recently two large Meta-analyses were performed[8-9].They provided robust evidence that there is no significant difference in IOP lowering effect between SLT and medication or between SLT and the more established argon laser trabeculoplasty (ALT). The mechanism by which SLT works shows some similarities to the mechanism by which IOP is decreased after lens extraction; release of several inf l ammatory mediators is responsible for lowering of the outf l ow resistance at the trabecular meshwork (TM). In SLT this is supposed to work by: 1) attraction of macrophages that clean up debris; 2)stimulating the formation of healthy TM tissue; 3) remodeling of the extracellular matrix of the TM[10-12].
In a retrospective study comparing SLT in pseudophakic and phakic patients, Shazly et al[13] recorded a delayed SLT response among pseudophakic patients at two weeks while the long-term effectiveness of SLT appeared the same in both groups.They argued that SLT and CCP may share a common pathway involving inf l ammation, prostaglandin release and interleukin release, which may be depleted after CCP, thus slowing down the response to SLT. Rosenfeld et al[14] compared the eff i cacy of SLT versus ALT in pseudophakic patients and found no significant differences in the IOP lowering effects between the two methods. Several retrospective studies compared the eff i cacy of SLT in pseudophakic and phakic eyes and found no signif i cant differences[15-18]. We know of no prior prospective clinical trial in the literature that compares the eff i cacy of SLT in pseudophakic and phakic eyes.
The purpose of this study was to examine the response to SLT in pseudophakic and phakic eyes in patients with POAG or ocular hypertension (OHT) in terms of IOP lowering effect,speed of response and possibility to decrease medication.
SUBJECTS AND METHODS
Study Design and Subjects Subgroup study of a larger prospective randomized clinical trial including 143 consecutive patients at the glaucoma consultation at Jan Palf i jn Hospital,Merksem, Belgium. Enrollment occurred from January 2014 to July 2015. The main goal of the study focused on the use of SLT in order to lower the amount of prescribed anti-glaucoma medication and examine effects of SLT on the quality of life.Approval of the Ethics Committee was obtained (EC 4313),we followed the guidelines of the Declaration of Helsinki.Trial registration information: SLT as replacement therapy in glaucoma patients, Nederlands trial register 5417. Data were recorded at baseline, at one hour, one week, one, three, six and twelve months post-SLT.
Inclusion criteria concerned POAG or OHT controlled with medical therapy. Only patients with recording of all data at all time points were included. Patients had to agree to sign an informed consent form. Exclusion criteria were other types of glaucoma than open angle glaucoma, previous fi ltering surgery or laser trabeculoplasty treatment. Patients with corneal disease that inhibited good visualization of the TM and patients on systemic steroids were also excluded from the study.
Of the original study, we extracted all pseudophakic eyes;38 eyes of 20 patients. All pseudophakic patients had their cataract removed through uneventful phacoemulsification with implantation of an intraocular lens in the capsular bag by the same surgeon under topical anesthesia at least one year before inclusion in the study. From the remaining 123 patients,we selected 38 eyes of 20 patients that were matched to the pseudophakic group in terms of demographic parameters (age,sex) and glaucoma parameters [baseline IOP with medication,type of glaucoma, central corneal thickness (CCT), cup disc ratio, visual fi eld mean def i cit, optical coherence tomography(OCT) focal loss of volume (FLV), IOP max before medication].This study was not designed to create additional IOP lowering effect, because IOP was already controlled with medication.Main goal of the original study was to maintain the same low IOP after SLT but with less medication and possibly improvement on the quality of life.
Baseline Examinations At baseline a full ophthalmological examination conducted, including a medical history review,best corrected visual acuity measurement, IOP measurement using Goldmann applanation tonometry (mean of two measurements was taken), slit lamp examination of the anterior segment (conjunctival injection, tear breakup time, cornea,iris, lens appearance, gonioscopy), CCT measurement, dilated fundus examination, visual fi eld examination by computerized perimetry (program 24-2, Humphrey Field Analyzer 745i,Zeiss, Jena, Germany), OCT of the optic disc and recording of glaucoma medications and artif i cial tears used prior to SLT.
All OCT scans were performed with the spectral-domain OCT RTVue (Optovue, Fremont, USA). We used FLV as determinant for the OCT (Zhang, American Glaucoma Society,Washington, March 1, 2014). Maximal IOP was calculated as the mean of three measurements taken at different time points before starting anti-glaucoma medication. IOP at baseline was calculated as the mean of the Goldmann measurements made on the three last examinations before laser treatment. The same examiner performed all examinations.
Laser Technique A frequency doubled, Q-switched Nd:YAG laser was used, emitting a wavelength of 532 nm, coupled to a slit-lamp delivery system (Lumenis Selecta Duet 5™). We used single pulses with a pulse duration of 3ns and spot size of 400 µm.Laser energy was initially set at 0.9 mJ and increased in steps of 0.1 mJ until minimal bubble formation was observed. We aimed to achieve minimal bubble formation during the whole treatment. All patients received 360° treatment of the TM. All treatments were applied by the same experienced surgeon (De Keyser M). Immediately before the laser procedure a drop of pilocarpine 1% and apraclonidine 0.5% were instilled in the treated eye. Immediately after the laser treatment, one drop of apraclonidine 0.5% was given in the treated eye. For the postoperative treatment patients were randomized for one of the 3 treatment regimen: dexamethasone drops 3 times daily,indomethacin collyre 3 times daily, or no drops. The second eye was treated one week later. All patients continued with the same anti-glaucomatous medical treatment after SLT.
Postoperative Management Patients were examined at 1h, 1wk, 1, 3, 6 and 12mo. At each visit, variables recorded included IOP, slit-lamp examination of anterior and posterior segment, subjective complaints, number of glaucoma drugs and artif i cial tears. Anti-glaucoma drops were continued until IOP was more than 2 mm Hg below target pressure, at which point they were stopped one by one. For example a latanoprost-timolol combination was considered as a combination of two separate medications; the first step entailed a switch to latanoprost if possible, the second step involved the discontinuation of the use of latanoprost at the next visit if possible. If IOP went above target IOP at any time point, medication was started again.
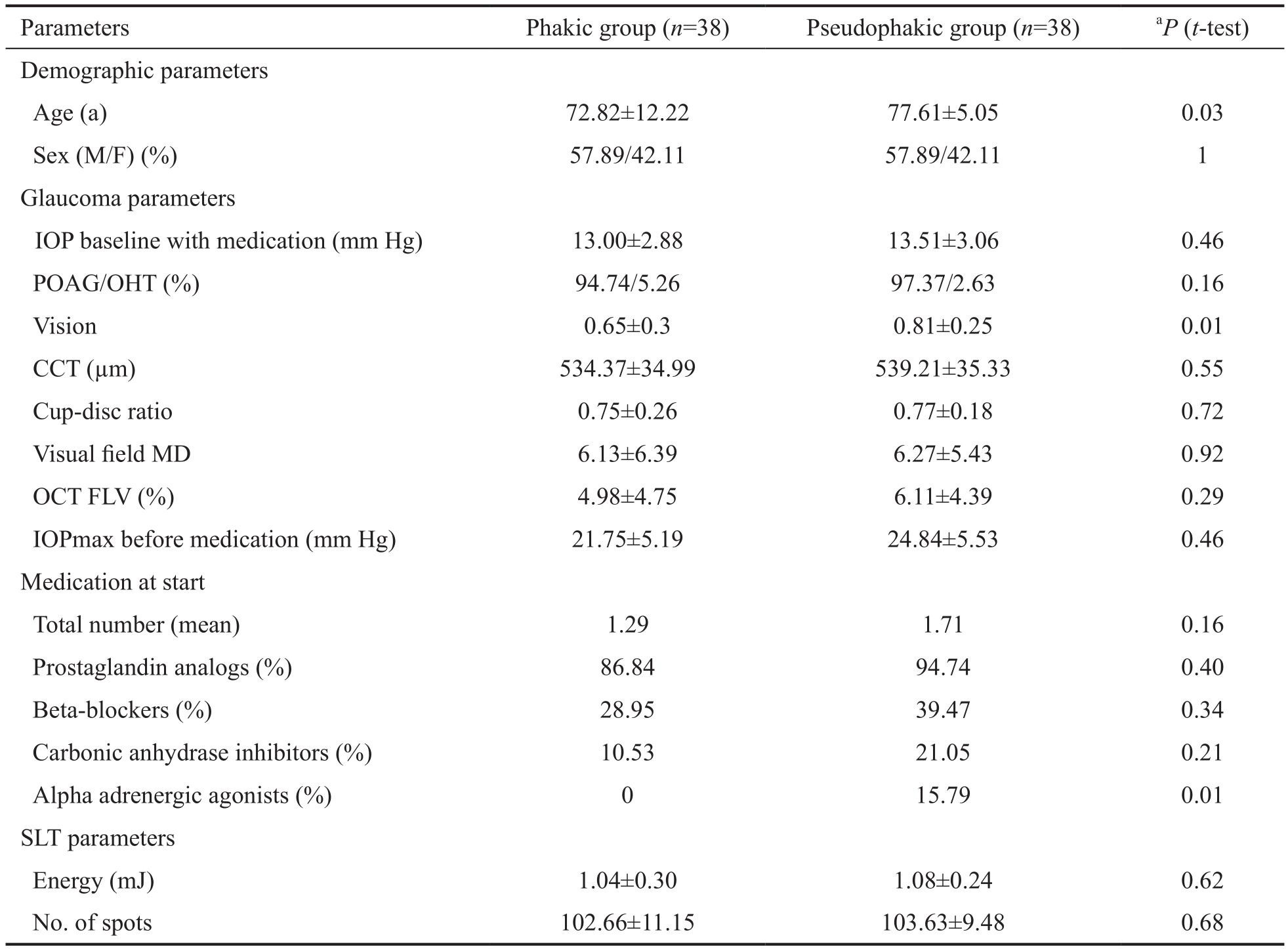
Table 1 Baseline characteristics of population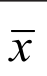 ±s
±s
IOP: Intraocular pressure; POAG: Primary open angle glaucoma; OHT: Ocular hypertension; CCT: Central corneal thickness;MD: Mean deviation in dB; OCT: Optical coherence tomography; FLV: Focal loss of volume. aStatistically signif i cant difference at Ρ<0.05.
Statistical Analysis An independent-samples t-test was performed to compare baseline differences between the phakic and pseudophakic group for continuous variables (e.g. age,IOP at medical baseline, vision, cup-disc ratio, CCT, visual fi eld mean def i cit, OCT FLV, IOP before treatment, number of medications at baseline). A generalizes linear model approach was applied to investigate the difference in evolution of mean IOP for both groups at all time points. To deal appropriately with ordinal and nominal data; an ordinal logistic regression was executed to investigate the time-evolution in number of used medications, with the overall effect of time on number of taken medications (5 time points) and between-subjects factor of patient group (phakic or pseudophakic). For both types of analysis values of the Wald Chi-square test are reported. In order to investigate differing proportions of fast-, slow- and non-responders between phakic and pseudophakic patients, a Chi-squared analysis for nominal data was performed. Results of statistical analysis with Ρ<0.05 were considered to be signif i cant.
RESULTS
Patient Demographics Baseline characteristics are shown in Table 1. Patients were matched for IOP at baseline, cup-disc ratio, CCT, visual fi eld mean def i cit and OCT FLV. Mean age in the pseudophakic group was higher (77.61±5.05y) than in the phakic group (72.82±12.22y). Visual acuity was better in the pseudophakic group (0.81±0.25) than in the phakic group(0.65±0.3) on Snellen chart. IOP at baseline was comparable between both groups (13.51±3.06 mm Hg in the pseudophakic,13.00±2.88 mm Hg in the phakic group), and low, as this was a population with controlled IOP under medication. At baseline 34 eyes of the phakic group (86.84%) and 36 of the pseudophakic group (94.74%) were taking prostaglandin analogs. Beta-blockers were used as second medication in 11 eyes of the phakic group (28.95%) and 15 of the pseudophakic group (39.47%). Only the use of alpha adrenergic agonists was different between the two groups. There is some debate about the influence on SLT outcome by carbo-anhydrase inhibitors and prostaglandin analogs[19-20], but alpha adrenergic agonists have not been found in clinical studies to affect SLT efficacy. All patients had a minimum follow up of 6mo, 24 eyes had a 12mo follow up in the phakic group, 29 in the pseudophakic group. All patients received 360° treatment of the TM; a mean of respectively 102.66±10.22 non-overlapping spots were placed in the phakic group and 103.63±9.48 in the pseudophakic group with a mean energy of respectively 1.04±0.30 and 1.08±0.24 mJ (Table 1).
Intraocular Pressure Evolution The mean IOP changed little in both groups. Baseline IOP was low in both groups as they were well controlled with medication. The aim of SLT was to decrease the number of medications needed, rather than have extra IOP lowering. The findings resulting from the generalized linear model approach demonstrated a nonsignif i cant interaction between time and IOP-evolution (Wald Chi-square=3.23, Ρ=0.78), demonstrating that the evolution in IOP over time did not differ between both patient groups.As a consequence, we were not allowed to perform any posthoc tests for each separate time point in order to compare both patient groups (Table 2).
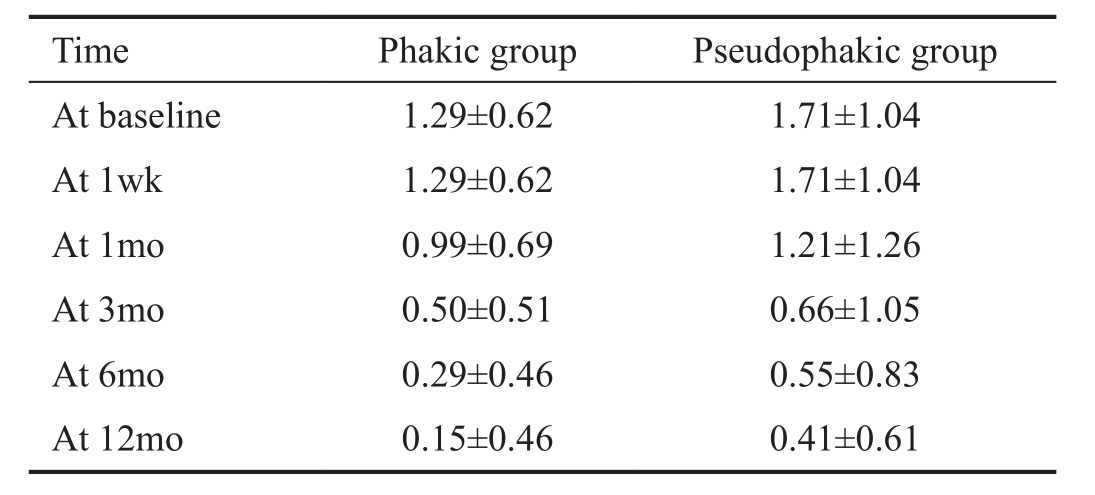
Number of Medications The number of glaucoma medications lowered in both groups (Wald Chi-square =163.47, Ρ<0.001)(Table 3). In the phakic group the number of medications changed from a mean of 1.29 at baseline to 0.15 after one year,or 88.37% reduction of medications. In the pseudophakic group the amount of medications lowered from a mean of 1.71 to 0.41,which entails a mean reduction of 76.02%. No interaction was observed between the patient groups and evolution in time(Wald Chi-square=4.00, Ρ=0.68); the reduction in number of medications in both groups was comparable.
Total success was def i ned as controlled IOP after SLT without further need of medication; this was achieved in 21 eyes(87.50%) of the phakic and 21 eyes of the pseudophakic group(72.41%). Qualified success was considered as IOP below target IOP with less medication than before SLT. This was reached in 100% of both the phakic and the pseudophakic eyes.
Speed of Response Patients who had an IOP more than 2 mm Hg below target IOP were told to lower their medication one by one. A second medication was stopped after a minimum wash out period of three months. The eyes in which medication could be diminished after check up at 1wk were considered fast responders. Those who could lower medication after 4 or 12wk were registered as slow responders. Those who could not lower their medication after 12wk were considered nonresponders.
In the phakic group we found 11 fast responders (29%), 26 slow responders (68.5%) and one non-responder (2.5%). The pseudophakic group contained 19 fast (50%), 17 slow (45%)and 2 non-responders (5%). Although these percentages suggest a larger number of fast responders in the pseudophakic group than in the phakic group, the differences between the two groups were not statistically significant (Wald Chisquare=4.35, Ρ=0.11).
Table 2 Mean IOP after SLT
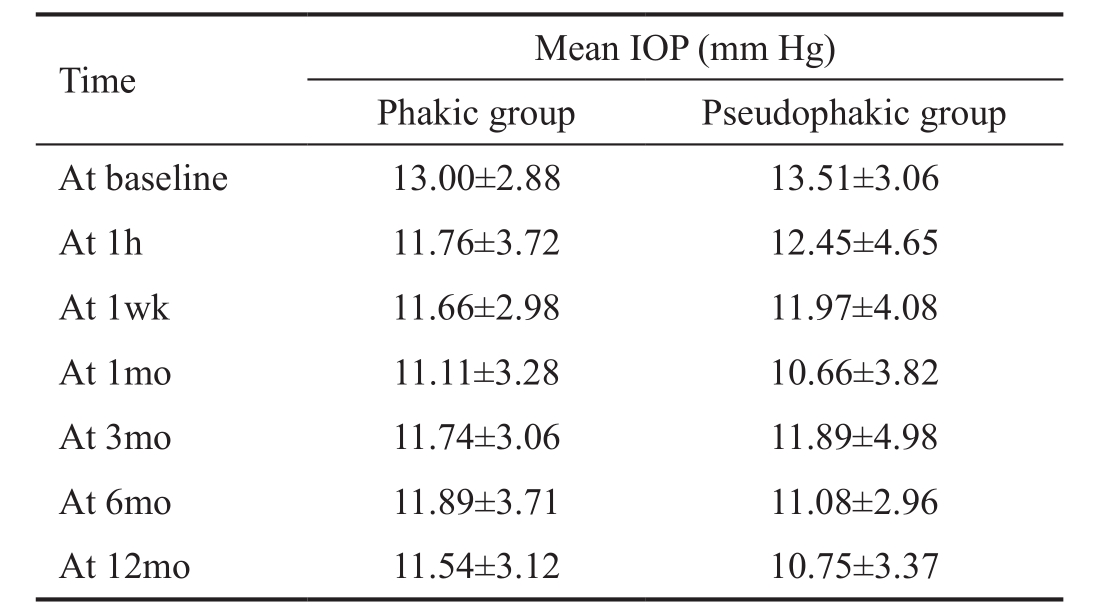
Table 3 Number of medications taken x±s
DISCUSSION
Cataract surgery can lead to a lowering of the IOP, both in normal[2-5]and glaucomatous eyes[4,6,21-23] but the physiopathology is still unclear. After lensextraction, increase of the anterior chamber depth and opening of the anterior chamber angle have been demonstrated[3,24]. Diminished production of aqueous by the ciliary body and enhanced outflow at the TM by flushing during surgery and following release of inflammatory mediators have also been proposed to contribute to the IOP lowering effect of cataract surgery[3].
SLT is a safe and efficient IOP lowering treatment[25-27]. It can be used as primary, adjunctive and replacement therapy in open angle glaucoma and OHT[8-9,28]. The mechanism of SLT is still unclear. SLT produces too little tissue changes to work mechanically, like ALT[29]. It is more likely that selective targeting of TM pigmented endothelial cells stimulates the production of interleukins that will attract macrophages who clean up debris at the TM[30]. On the other hand a cellular mechanism enhancing rejuvenation of the trabeculum has been found[11-12].
If SLT would work through the same mechanism as phaco emulsif i cation to lower IOP, it could be expected that the IOP lowering effect of SLT would be lower in pseudophakic eyes because the mechanisms have been depleted partially or the pathways have already been activated.
Lindegger et al[31] measured more IOP reduction in a phakic group compared to pseudophakic eyes at one month. They did however not demonstrate a signif i cant difference at any other time point (1d, 1, 3mo and every 3mo up to 43mo) Shazly et al[13] reported that IOP reduction following SLT was higher in phakic than in pseudophakic eyes 2wk after SLT, but reached the same level at 3mo and remained comparable for the entire follow up of 30mo. Several retrospective studies(ranging from 18[16] to 40[31] pseudophakic and 21[32] to 113[31]phakic eyes) have also compared the IOP lowering effect of SLT in pseudophakic and phakic eyes: the groups op Werner et al[16], Kalbag et al[17] and Seymenoglu and Baser[15] found no signif i cant difference in SLT eff i cacy or success rates between phakic and pseudophakic eyes.The same findings were reported in several studies by Lee et al[33-35]. Our study is in keeping with these studies; we found no signif i cant differences between the pseudophakic and the phakic patients at any time point up to a follow up of one year. IOP lowering effect was comparable, as was the decrease in number of medications needed.
Speed of Selective Laser Trabeculoplasty Effect Nagar et al[36]noted that IOP lowering effect after SLT is predominantly immediate; lower IOP after one week. Her group recorded 10%-15% of slow responders, whose IOP lowering only occurred 4 to 12wk after SLT. We can conf i rm the presence of fast and slow responders. We found more fast responders in the pseudophakic group (50%) than in the phakic group (29%), the difference was however not signif i cant (Ρ>0.05). In the phakic group, reaction to SLT was slower in 71% of the patients.Therefore, one should always wait 3mo to evaluate the full effect of SLT.
The major limitation of our study is the limited follow up period of 12mo. However, previous investigators have shown that the IOP lowering effect of SLT after cataract extraction persists for at least 24mo[22]. The second limitation is the limited number of eyes (76) examined. More extensive prospective investigation is needed. There was no signif i cant difference between pseudophakic and phakic eyes in terms of IOP lowering effect and decrease of medication needed. Within one week after SLT, 40% of the eyes responded with IOP lowering, 57% responded after 1 to 3mo. At least 3mo time has to be given before drawing conclusions on the IOP lowering effect after SLT.
ACKNOWLEDGEMENTS
Conflicts of Interest: De Keyser M, None; De Belder M,None; De Groot V, None.
REFERENCES
1 Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R,Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82(11):844-851.
2 Bhallil S, Andalloussi IB, Chraibi F, Daoudi K, Tahri H. Changes in intraocular pressure after clear corneal phacoemulsification in normal patients. Oman J Ophthalmol 2009;2(3):111-113.
3 Issa SA, Pacheco J, Mahmood U, Nolan J, Beatty S. A novel index for predicting intraocular pressure reduction following cataract surgery. Br J Ophthalmol 2005;89(5):543-546.
4 Jamil AZ, Iqbal K, Ur Rahman F, Mirza KA. Effect of phacoemulsification on intraocular pressure. J Coll Ρhysicians Surg Ρak 2011;21(6):347-350.
5 Mannes K, Zeyen T. Reduction in IOP after clear corneal phacoemulsif i cation in normal patients. Bull Soc Belge Ophtalmol 2001;282:19-23.6 Inal A, Bayraktar S, Inal B, Bayraktar Z, Yilmaz OF. Intraocular pressure control after clear corneal phacoemulsification in eyes with previous trabeculectomy: a controlled study. Acta Ophthalmol Scand 2005;83(5):554-560.
7 Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-κB response: a potential mechanism for intraocular pressure reduction after phacoemulsif i cation. Invest Ophthalmol Vis Sci 2003;44(5):1977-1981.
8 Li X, Wang W, Zhang X. Meta-analysis of selective laser trabeculoplasty versus topical medication in the treatment of open-angle glaucoma. BMC Ophthalmol 2015;15:107.
9 Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the eff i cacy of selective laser trabeculoplasty in openangle glaucoma. Surv Ophthalmol 2015;60(1):36-50.
10 Damji KF, Shah KC, Rock WJ, Bains HS, Hodge WG. Selective laser trabeculoplasty v argon laser trabeculoplasty: a prospective randomised clinical trial. Br J Ophthalmol 1999;83(6):718-722.
11 Bylsma SS, Samples JR, Acott TS, Van Buskirk EM. Trabecular cell division after argon laser trabeculoplasty. Arch Ophthalmol 1988;106(4):544-547.
12 Bradley JM, Anderssohn AM, Colvis CM, Parshley DE, Zhu XH,Rudddat MS, Samples JR, Acott TS. Mediation of laser trabeculoplastyinduced matrix metalloproteinase expression by IL-1beta and TNFalpha.Invest Ophthalmol Vis Sci 2000;41(2):422-430.
13 Shazly TA, Latina MA, Dagianis JJ, Chitturi S. Effect of prior cataract surgery on the long-term outcome of selective laser trabeculoplasty. Clin Ophthalmol 2011;5:377-380.
14 Rosenfeld E, Shemesh G, Kurtz S. The efficacy of selective laser trabeculoplasty versus argon laser trabeculoplasty in pseudophakic glaucoma patients. Clin Ophthalmol 2012;6:1935-1940.
15 Seymenoglu G, Baser EF. Eff i cacy of selective laser trabeculoplasty in phakic and pseudophakic eyes. J Glaucoma 2015;24(2):105-110.
16 Werner M, Smith MF, Doyle JW. Selective laser trabeculoplasty in phakic and pseudophakic eyes. Ophthalmic Surg Lasers Imaging 2007;38(3):182-188.
17 Kalbag N, Patel S, Khouri A, Berezina T, Fechtner R, Cohen A.Selective laser trabeculoplasty in the treatment of glaucoma in phakic versus pseudophakic patients. Invest Ophthalmol Vis Sci 2013;54(15):1862.
18 Mahdaviani S, Kitnarong N, Kropf JK, Netland PA. Eff i cacy of laser trabeculoplasty in phakic and pseudophakic patients with primary openangle glaucoma. Ophthalmic Surg Lasers Imaging 2006;37(5):394-398.
19 Lai JS, Chua JK, Tham CC, Lam DS. Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Experiment Ophthalmol 2004;32(4):368-372.
20 Singh D, Coote MA, O’Hare F, Walland MJ, Ghosh S, Xie J, Ruddle JB, Crowston JG. Topical prostaglandin analogues do not affect selective lasertrabeculoplasty outcomes. Eye (Lond) 2009;23(12):2194-2199.
21 Leelachaikul Y, Euswas A. Long-term intraocular pressure change after clear corneal phacoemulsi fi cation in Thai glaucoma patients. Chotmaihet thangphaet 2005;88 (Suppl 9):S21-S25.
22 Mathalone N, Hyams M, Neiman S, Buckman G, Hod Y, Geyer O.Long-term intraocular pressure control after clear corneal phacoemulsifi cation in glaucoma patients. J Cataract Refract Surg 2005;31(3):479-483.
23 Picoto M, Galveia J, Almeida A, Patrício S, Spohr H, Vieira P, Vaz F. Intraocular pressure (IOP) after cataract extraction surgery. Revista Brasileira de Oftalmologia 2014;73(4):230-236.
24 Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology 2000;107(4):698-703.
25 Lee JY, Lee YK, Moon JI, Park MH. Long-term outcomes and predictive factors for success of selective laser trabeculoplasty. J Korean Ophthalmol Soc 2014;55(9):1347.
26 Ayala M, Chen E. Long-term outcomes of selective laser trabeculoplasty(SLT) treatment. Open Ophthalmol J 2011;5(1):32-34.
27 Shazly TA, Latina MA. Intraocular pressure response to selective laser trabeculoplasty in the fi rst treated eye vs the fellow eye. Arch Ophthalmol 2011;129(6):699-702.
28 McIlraith I, Strasfeld M, Colev G, Hutnik CM. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma 2006;15(2):124-130.
29 Cvenkel B, Hvala A, Drnovek-Olup B, Gale N. Acute ultrastructural changes of the trabecular meshwork after selective laser trabeculoplasty and low power argon laser trabeculoplasty. Lasers Surg Med 2003;33(3):204-208.
30 Detorakis ET, Tsiklis N, Pallikaris IG, Tsilimbaris MK. Changes in the intraocular pressure of fellow untreated eyes following uncomplicated trabeculectomy. Ophthalmic Surg Lasers Imaging 2011;42(2):138-143.31 Lindegger DJ, Jaggi GP, Bauer G, Zweifel S, Toteberg-Harms M, Hirn C, Zehnder S, Funk J. Long-term efficacy of selective laser trabeculoplasty in pseudophakic patients-a “real-life” analysis. Klin Monbl Augenheilkd 2014;231(4):357-362.
32 Abdelrahman A, Eltanamly R. Selective laser trabeculoplasty in pseudophakic patients with open angle glaucoma. J Egypt Ophthalmol Soc 2014;107(4):268.
33 Lee JW, Liu CC, Chan JC, Lai JS. Predictors of success in selective laser trabeculoplasty for normal tension glaucoma. Medicine (Baltimore)2014;93(28):e236.
34 Lee JW, Liu CC, Chan JC, Lai JS. Predictors of success in selective laser trabeculoplasty for Chinese open-angle glaucoma. J Glaucoma 2014;23(5):321-325.
35 Lee JW, Liu CC, Chan JC, Wong RL, Wong IY, Lai JS. Predictors of success in selective laser trabeculoplasty for primary open angle glaucoma in Chinese. Clin Ophthalmol 2014:8:1787-1791.
36 Nagar M, Luhishi E, Shah N. Intraocular pressure control and fl uctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol 2009;93(4):497-501.