
·Clinical Research·Current
Issue· ·Achieve· ·Search Articles· ·Online Submission· ·About IJO· PMC
Analyzing cytokines as biomarkers
to evaluate severity of glaucoma
Yao Tong1,2, Ya-Li Zhou1, Yan Zheng2,3,
Manas Biswal4, Pei-Quan Zhao2, Zhao-Yang Wang1
1Department of Ophthalmology,
Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of
Medicine, Shanghai 200011, China
2Department of
Ophthalmology, Xinhua Hospital Affiliated to Shanghai Jiaotong University
School of Medicine, Shanghai 200092, China
3Department of
Ophthalmology, Xinhua Hospital Affiliated to Shanghai Jiaotong University
School of Medicine, Chongming Branch, Shanghai 202150, China
4Department of Molecular
Genetics, University of Florida, Gainesville, Florida 32610, USA
Correspondence
to: Zhao-Yang
Wang. Department of Ophthalmology, Shanghai Ninth People’s Hospital, Shanghai
Jiaotong University School of Medicine, Shanghai 200011, China.
zhaokekewzy@hotmail.com; Yan Zheng. Department of Ophthalmology, Xinhua
Hospital affiliated to Shanghai Jiaotong University School of Medicine,
Shanghai 200092, China. clairvoyant@126.com
Received:
2016-09-28
Accepted: 2017-02-06
Abstract
AIM: To analyze
cytokines as biomarkers for evaluation of severity of glaucoma.
METHODS: This was a
prospective case-control study including 29 eyes with glaucoma. Besides, 28
eyes with senile cataract were used as control. Patients were classified into
four groups: acute angle closure glaucoma (AACG), chronic angle closure
glaucoma (CACG), primary open angle glaucoma (POAG) and senile cataract.
Undiluted vitreous samples were collected, then vitreous concentrations of 9
types of cytokines were determined by cytometric bead assay system: γ-interferon
(IFNg), interleukin (IL)-10, IL-2, IL-4, IL-5, interferon-γ-inducible
protein (IP)-10, monocyte chemoattractant protein (MCP)-1, tumor necrosis
factor (TNF) -α, and vascular
endothelial growth factor (VEGF). We also recorded the intraocular pressure
(IOP) of patients in each group and Pearson correlated analysis was performed
to analysis the correlation between each type of cytokine with IOP.
RESULTS: Vitreous levels
of IL-2, IL-5, MCP-1, TNF-α and IP-10 were
significantly higher (P<0.05) in AACG group. Patients with AACG, CACG
and POAG have higher IOP than senile cataract, but we didn’t find any
significant correlation between IOP with any type of the cytokines.
CONCLUSION: Inflammation and
immune reaction have a strong link with the pathology of glaucoma especially
AACG. Some cytokines may act as biomarkers to evaluate the severity of
glaucoma. Anti-inflammatory treatments and controlling of IOP are necessary for
the therapy of glaucoma.
KEYWORDS: glaucoma; cytokines;
intraocular pressure
DOI:10.18240/ijo.2017.06.15
Citation:Tong Y, Zhou YL, Zheng Y, Biswal M, Zhao PQ, Wang ZY. Analyzing cytokines as biomarkers to evaluate severity
of glaucoma. Int J Ophthalmol 2017;10(6):925-930
Article
Outline
INTRODUCTION
Glaucoma is
one of the main causes of blindness worldwide. The elevated intraocular pressure
(IOP) is the main risk factor while it is also characterized by a progressive
glaucomatous optic neuropathy and corresponding visual field loss[1-2]. Meanwhile, some studies have
suggested that glaucoma actually involves multiple factors, including immune
reactions[3], inflammation[4],
ischemia[5], hypoxia[6],
and oxidative stress[7]. Glaucoma can be
categorized into acute angle closure glaucoma (AACG), chronic angle closure
glaucoma (CACG), primary open angle glaucoma (POAG) depending on the different
pathogenesis.
The role of
immunological factors in glaucoma has become a major research topic recently
and cytokines mediate immune and inflammatory responses may play an important
role in the process of glaucomatous optic neuropathy[8].
Previous studies that measured cytokine concentrations in aqueous humor samples
have detected elevated cytokine levels in eyes suffering from glaucoma, such as
interleukin (IL)-9, 10, 12, interferon (IFN)-α, γ, monokine induced by IFN-γ
(MIG or CXCL9), etc[9-13].
T-helper (Th) cells are the main source of cytokines, and some studies
suggested that balance of Th1/Th2 cytokines plays an important role in the
mechanism of glaucomatous optic neuropathy[14-15]. Vascular endothelial growth factor (VEGF) may also
play an important role in the mechanism of neovascular glaucoma (NVG)[16], which is a kind of cytokine that could promote
neovascularization and has a strong link with inflammation and immunity[17]. The evidence from these previous studies indicates
that the levels of cytokines in aqueous humor (AH) may be related to the
pathogenesis of glaucoma. Therefore, evaluation of those cytokines in AH may
expand the understanding of glaucoma pathophysiology.
Cytometric
bead assay has greater sensitivity than traditional enzyme-linked immunosorbent
assay (ELISA) and spots enzyme immunoassay (elispots), it allows simultaneous
detection of multiple cytokines in a small volume clinical samples[18]. This technique has been successfully used to detect
the levels of several cytokines in AH of patients with active panuveitis and
anterior uveitis (AU) in other study[19]. In our
study, we measured multiple cytokines in the AH of eyes with AACG, CACG, POAG
and senile cataract using cytometric bead assay to investigate the possible
roles of the intraocular cytokines in the pathogenesis of glaucoma.
SUBJECTS AND METHODS
This study was
performed in accordance with the tenets of Declaration of Helsinki, and the
produces were approved by the Institutional Review Board of Xinhua Hospital.
All the patients signed a written informed consent after an explanation of
nature and possible consequences of this study. All the samples were collected
from April 2014 to January 2015.
Subjects Patient’s inclusion criteria: 1)
patients have been diagnosed as any type of glaucoma and need filtering surgery
therapy; 2) patients has no filtering operation therapy history before; 3)
patients’ age and medical history were clear; 4) patients have signed the
informed consent.
Patient’s
exclusion criteria: 1) patients have other ophthalmic diseases; 2) patients
have other systemic diseases; 3) patients have accepted systemic and local
steroids therapy in one week; 4) patients have accepted ophthalmic surgery or
intravitreous injections before.
Twenty-nine
eyes of twenty-nine patients with glaucoma (8 patients with AACG, 15 patients
with CACG and 6 patients with POAG) were studied. Besides, twenty-eight eyes of
twenty-eight patients with senile cataract and have no history of IOP exceeding
21 mm Hg were used as control.
At last,
patients were classified into four groups: AACG group, CACG group, POAG group
and senile cataract group.
Sample
Collection All the patients with
glaucoma underwent filtering surgery at Xinhua Hospital while the patients with
cataract underwent phaco+IOL surgery. IOP was recorded for patients in each
group. After anesthesia, 0.1 mL AH in anterior chamber was collected through a
lateral hyal-corneal incision by using a 30-gauge needle connected with 1 mL
syringe before the surgery. The needle was not contact with iris, lens or
corneal endothelium. The collected vitreous samples were stored at -80℃ until
the assay validation.
Cytometric
Bead Assay The vitreous levels of 9
types of cytokines were determined simultaneously by a commercially available
cytometric bead assay (Becton, Dickinson and Company). Analyses were performed
for γ-interferon (IFNg), IL-10, IL-2, IL-4, IL-5, interferon-γ-inducible
protein (IP)-10, monocyte chemoattractant protein (MCP)-1, tumor necrosis
factor (TNF) -α, and VEGF.
The samples
were thawed in room temperature, then centrifuged at 15 000 rpm for 10min at 4℃.
The 50 μL of each sample and different concentrations of each cytokine standard
were added to 50 μL antibody-conjugated beads in a 96-well filter plate. After
30min incubation, the plate was washed and after another 30min of incubation,
25 μL biotinylated antibody solution was added to each well. Then washed the
plates, and 50 μL streptavidin-conjugated phycoerythrin was added to each well
and incubated for 10min. After a final wash, the contents of each well were
resuspended in 125 μL assay buffer and were analyzed using a BD Bead Array
Reader. The concentrations of the cytokines were calculated from a standard
curve for each cytokine.
Statistical
Analysis Statistical analysis was
performed using SPSS 13.0 software. Data was presented as average and range. If
P-value of the homogeneity of variance >0.05, a one way-AVONA
analysis was used to detect the differences of the vitreous concentrations of
each cytokine among AACG; CACG; POAG and senile cataract group, followed by
Bonferroni test to detect the differences between each two groups. If P-value
of the homogeneity of variance <0.05, a Kruskal-Wallis 1-way analysis was
performed to test the differences for the vitreous concentrations of each
cytokine among those groups, followed by Tamhane’s T2 to detect the differences
between each two groups. The correlation between two parameters was determined
by Pearson correlation. A P-value <0.05 was considered to be
statistically significant.
RESULTS
The sample
sizes and average ages of each group are shown in Table 1. The average age is
66.25 in AACG group; 70.77 in CACG group; 63.33 in POAG group and 72.75 in
senile cataract group.
Table 1
Demographics of cases in each groups
|
Parameters |
n |
Age (a) |
|
AACG |
8 |
66.25 (47-94) |
|
CACG |
15 |
70.77 (50-95) |
|
POAG |
6 |
63.33 (50-82) |
|
Senile
cataract |
28 |
72.75 (50-84) |
AACG: Acute
angle closure glaucoma; CACG: Chronic angle closure glaucoma; POAG: Primary open
angle glaucoma.
Vitreous
Levels of 9 Types of Cytokines The vitreous levels of
IL-10, IL-2, IL-5, IP-10, MCP-1, TNF-α and VEGF were significantly different
(all P<0.001) among AACG, CACG, POAG and senile cataract group. We
also did comparison between each two groups. There is no significant difference
of IL-10 or VEGF between each two groups. The vitreous level of IL-2 was
significantly higher in AACG group than CACG group (P=0.036), POAG group
(P=0.044) and senile cataract group (P=0.031). The vitreous level
of IL-5 was significantly higher in AACG group than CACG group (P<0.001),
POAG group (P<0.001) and senile cataract group (P<0.001).
The vitreous level of IP-10 was significantly higher in AACG group than CACG
group (P=0.012) and senile cataract group (P=0.004). The vitreous
level of MCP-1 was significantly higher in AACG group than CACG group (P<0.001),
POAG group (P<0.001) and senile cataract group (P<0.001).
The vitreous level of TNF-α was significantly higher in AACG group than CACG
group (P=0.003), POAG group (P=0.002) and senile cataract group (P=0.002)
(Table 2). Figure 1 shows the scatter plots of each type of cytokine in each
group.
Table 2
Vitreous levels of 9 types of cytokines in eyes with AACG, CACG, POAG, senile
cataract
|
Parameters |
AACG (n=8) |
CACG (n=15) |
POAG (n=6) |
Senile cataract (n=28) |
P |
|
IFNg |
1.36 (1.08-1.82) |
1.27 (0.98-1.52) |
1.20 (1.08-1.30) |
1.29 (0.98-1.70) |
0.434a |
|
IL-10 |
3.43 (1.01-8.90) |
0.25 (0.14-0.60) |
0.25 (0.18-0.37) |
0.35 (0.10-2.63) |
<0.001b |
|
IL-2 |
1.35 (0.55-2.36)d |
0.42 (0.35-0.55)d |
0.46 (0.35-0.53)d |
0.39 (0.26-0.48)d |
<0.001b |
|
IL-4 |
0.18 (0.11-0.30) |
0.13 (0.04-0.22) |
0.14 (0.09-0.22) |
0.13 (0.05-0.24) |
0.069a |
|
IL-5 |
0.74 (0.53-0.91)c |
0.55 (0.43-0.88)c |
0.48 (0.37-0.56)c |
0.55 (0.43-0.68)c |
<0.001a |
|
IP-10 |
1758.88 (965-3292)d |
594.20 (239-2105)d |
713.33 (307-2025) |
251.72 (30-653)d |
<0.001b |
|
MCP-1 |
19611 (13695-24413)c |
4255.87 (1150-12169)c |
4930.17 (2848-12436)c |
3608.32 (1209-10330)c |
<0.001a |
|
TNF-α |
3.60 (2.49-4.94)d |
2.13 (1.59-3.12)d |
2.10 (1.76-2.56)d |
2.04 (1.53-2.47)d |
<0.001b |
|
VEGF |
1872.25 (117-7659) |
300.17 (35.78-1536) |
86.40 (1.79-362) |
158.34 (1.79-448) |
<0.001b |
AACG: Acute angle
closure glaucoma; CACG: Chronic angle closure glaucoma; POAG: Primary open
angle glaucoma; IFNg: γ-interferon; IL: Interleukin; IP: Interferon-γ-inducible
protein; MCP: Monocyte chemoattractant protein; TNF: Tumor necrosis factor;
VEGF: Vascular endothelial growth factor. Levels are expressed as the average
(range) pg/mL. aOne way-AVONA analysis was performed to compare the
four groups; bKruskal-Wallis 1-way analysis was performed to compare
the four groups; Significant (P<0.05) difference for comparison
versus control: cBonferroni test and dTamhane’s T2.
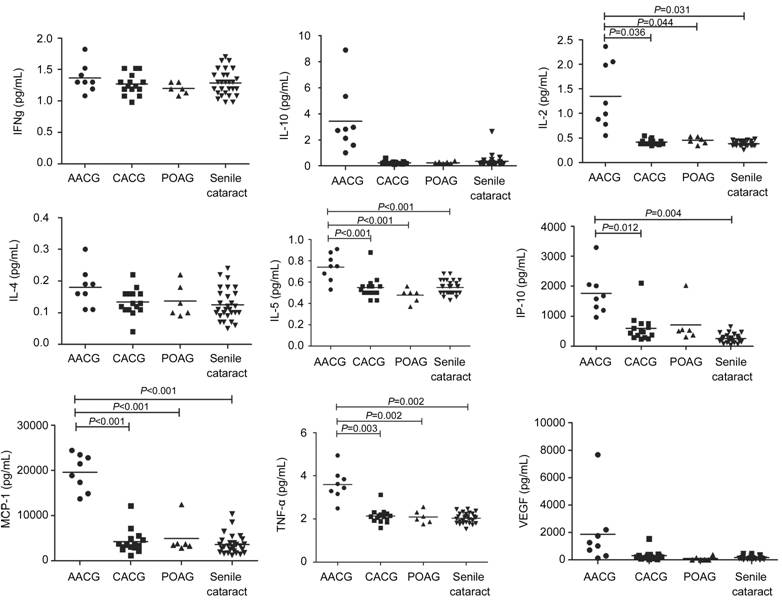
Figure 1
Scatter plots: distribution levels of 9 types of cytokines in eyes with AACG,
CACG, POAG and senile cataract.
Correlation
Analysis We recorded IOP for
patients in each group (Table 3) and Pearson correlated analysis was performed
to check the correlation between each type of cytokine with IOP (Table 4).
Patients with AACG, CACG and POAG have higher IOP than senile cataract, but
because of the small sample size, only the difference between CACG group and
senile cataract group is significant. Meanwhile, we didn’t find any significant
correlation between any type of cytokine with IOP in those group.
Table 3 IOP
of eyes with AACG, CACG, POAG and senile cataract
|
Parameters |
AACG |
CACG |
POAG |
Senile cataract |
P |
|
n |
5 |
15 |
6 |
27 |
|
|
IOP (mm Hg) |
29.74 (18.9-39.3) |
27.33 (9-52)b |
28.55 (16-42.3) |
14.63 (9.8-19.5)b |
<0.001a |
AACG: Acute
angle closure glaucoma; CACG: Chronic angle closure glaucoma; POAG: Primary open
angle glaucoma; IOP: Introccular pressure. Levels are expressed as the average
(range) mm Hg. aKruskal-Wallis 1-way analysis was performed to
compare the four groups; Significant (P<0.05) difference for
comparison versus control: bTamhane’s T2.
Table 4
Correlations between cytokines and IOP
|
Parameters |
AACG |
CACG |
POAG |
|||
|
ρ |
P |
ρ |
P |
ρ |
P |
|
|
IFNg |
0.638 |
0.246 |
-0.008 |
0.979 |
-0.759 |
0.08 |
|
IL-10 |
0.431 |
0.469 |
0.158 |
0.573 |
0.346 |
0.502 |
|
IL-2 |
-0.122 |
0.845 |
0.299 |
0.279 |
-0.133 |
0.801 |
|
IL-4 |
0.226 |
0.715 |
0.042 |
0.882 |
0.523 |
0.287 |
|
IL-5 |
0.196 |
0.752 |
-0.365 |
0.18 |
0.64 |
0.171 |
|
IP-10 |
0.761 |
0.135 |
0.208 |
0.456 |
-0.001 |
0.999 |
|
MCP-1 |
-0.4 |
0.505 |
0.194 |
0.488 |
0.014 |
0.978 |
|
TNF-α |
-0.141 |
0.822 |
0.334 |
0.223 |
0.457 |
0.362 |
|
VEGF |
-0.146 |
0.814 |
0.29 |
0.295 |
-0.054 |
0.919 |
AACG: Acute
angle closure glaucoma; CACG: Chronic angle closure glaucoma; POAG: Primary
open angle glaucoma; Pearson correlation (ρ) and P value were calculated
by pearson correlation and a significant difference was accepted when P<0.05.
DISCUSSION
Levels of 9 different cytokines were
analyzed simultaneously in 0.1 mL AH. Although the sample volume is very small,
cytometric bead assay has great sensitivity and also allows for simultaneous
detection of multiple cytokines in small volume clinical samples.
We included
patients with AACG, CACG, POAG and detected cytokines for each group to see if
different pathogenic mechanisms could impact the vitreous levels of cytokines.
The result shows that IL-2, IL-5, MCP-1, TNF-α and IP-10 were significantly
higher in AACG group. However, there is no significant difference was found
among any other groups. IL-2 induces T-cell proliferation and affects the levels
and function of cytotoxic and regulatory T cells as well as the production of
antibodies. Hou et al[20] found
significant mRNA elevation for IL-2 on the iris of patients with neovascular
glaucoma. TNF-α is a kind of pleiotropic cytokine which has many physiological
functions. A study showed that TNF-α which produced by retinal glial cells is
one of the risk factors for glaucoma[21].
Upregulation of expression of TNF-α and its receptor TNF-R1 can induce
apoptosis of retinal ganglion cells. In the rat model of high IOP induced by
laser photocoagulation, the expression of TNF-R1 gene was 8 times higher than
the normal rat, the level of TNF-α was also significantly increased[22]. Another study proved that adding anti TNF-α
antibodies or TNF-R1 conditioned medium of ischemia, retinal ganglion cell
death are greatly reduced[23]. Tezel and Wax[24] found that anti TNF-α antibodies can lead to
decreased rate of retinal ganglion cells apoptosis by about 66%. Xin et al’s[25] study showed that patients with open angle glaucoma
have higher TNF-α levels in AH compared with the control subjects. IP-10
belongs to the CXC chemokine family which can induce chemotaxis, cell growth,
apoptosis, angiogenesis, and inflammation mediated by combining with the CXC
chemokine receptor 3 (CXCR3) and also involved in the formation of inflammatory
and immune responses to infection and tumor. Studies have shown that IP-10 is
related to diabetic macular edema, neovascular macular degeneration and
polypoidal choroidal vasculopathy[26-27],
but the relationship between IP-10 and glaucoma is still unreported. IL-5 and
MCP-1 are also involved in the inflammatory and immune responses in many
situations and higher level of MCP-1 in eyes with glaucoma was proved by Huang et
al[28].
There are
other previous studies test the concentrations of different cytokines in eyes
with glaucoma. Huang et al[28] found
elevated concentrations of IL-6, IL-8, granulocyte colony-stimulating factor
(G-CSF), MCP-1, MCP-3, and VEGF in eyes with AACG. Chua et al’s[9] study certificated that concentration of IL-9, IL-12,
IFN-α, IFN-γ, CXCL9 and IL-10 are higher in eyes with glaucoma and especially
eyes with POAG have higher IL-12 , IFN-γ and CXCL9 levels, while eyes with PACG
had higher interleukin-8 (CXCL8) and CXCL9 levels. In Takai et al’s[29] study, the concentrations of IL-8, transforming
growth factor (TGF)-1, and serum amyloid A (SAA) were significantly higher and
IL-6 was significantly lower in the eyes with POAG. Borkenstein et al[10] also found a lower level of IL-6 in eyes with POAG.
The previous studies together with our study all show that immune and
inflammatory responses may have a close relationship with the pathology of
glaucoma. IL-2, IL-5, IL-10, MCP-1, IP-10 and TNF-α can mediate immune and
inflammatory responses in lots of situations. Among those 6 cytokines, IL-2,
TNF-α are regulated by Th1 cells while IL-5, IL-10 are regulated by Th2 cells.
Some studies considered that imbalance of Th1/Th2 are associated with many
diseases, such as allergies, tumor progression, graft rejection and so on[30-33]. What we found in our study
suggesting that imbalance of Th1/Th2 cytokines could also play an important
role in the mechanism of glaucomatous optic neuropathy.
However, the
specific reason and mechanism of the relationship between high levels of
cytokines and glaucoma are still uncertain. The elevated levels of cytokines
may caused by the development of glaucoma and are the results of the acute crisis.
Different pathogenesis may lead to different levels of cytokines. The
concentrations of the cytokines are also possibly influenced by the use of
medicine since our study did not detect the relationship between the cytokines
and medicine which used by the patients to control the symptoms of glaucoma.
Thus, more researches should be performed to study the basic mechanisms and the
reasons of the high levels of cytokines in glaucoma eyes.
We didn’t
detect any significant correlation between those cytokines with IOP, the reason
is probably because our sample size is small. However, Freedman and Iserovich’s[33] study shows that levels of intraocular cytokines
increase with an increase in IOP. Huang et al[28]
also found that IOP may be responsible for the production of cytokines in
eyes with AACG and elevated cytokine levels may in turn influence AH dynamics
and thus lead to IOP elevation. Takai et al’s[29]
study showed that cytokine networks including TGF-1, IL-8, and SAA in AH may
have critical roles in IOP elevations in patients with POAG. The results of
these studies suggest that anti-inflammatory treatments are necessary for
controlling IOP in eyes suffering from glaucoma.
Another
limitation of our study is the sample size. It is too small to get significant
results for some groups. Besides, during the sample collection, any possible
contamination may affect the result even if we tried to avoid it. Therefore,
more precise further studies with a larger sample size are still needed to
detect the relationship between inflammation and immune with different kinds of
glaucoma and the relationship between different cytokines with IOP.
In conclusion,
the significant results of our study suggest that inflammation and immune
reaction have a strong link with the pathogenesis of glaucoma especially AACG.
Some cytokines may act as biomarkers to evaluate the severity of glaucoma.
Anti-inflammatory treatments and controlling of IOP are very necessary for the
therapy of glaucoma.
ACKNOWLEDGEMENTS
Authors’
Contributions: Wang ZY and Zheng Y
conceived and designed the study. Tong Y, Zhou
YL, Wang ZY, and Zheng
Y collected the samples. Tong Y and Zhou YL performed the data statics and
analysis. Tong Y wrote the paper. Biswal
M and Zhao PQ
reviewed and edited the manuscript.
Foundations:
Supported
by the National Natural Science Fundation of China (No.81371040); Shanghai
Pujiang Program (No.15PJD028); Scientific Research Project of Shanghai
Municipal Health Bureau (No.20124149).
Conflicts
of Interest: Tong Y, None; Zhou YL, None; Zheng Y, None; Biswal M, None;
Zhao PQ, None; Wang ZY, None.
REFERENCES
1 Gupta N, Weinreb RN. New definitions
of glaucoma. Curr Opin Ophthalmol 1997;8(2):38-41. [CrossRef] [PubMed]
2 Sommer A. Intraocular pressure and
glaucoma. Am J Ophthalmol
1989;107(2):186-188. [CrossRef]
3 Tezel G, Wax MB. The immune system and
glaucoma. Curr Opin Ophthalmol
2004;15(2):80-84. [CrossRef] [PubMed]
4 Li G, Luna C,
Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after
oxidative stress in trabecular meshwork cells. Mol Vis 2007;13:2282-2288. [PMC free article] [PubMed]
5 Nakabayashi M. Review of the ischemia
hypothesis for ocular hypertension other than congenital glaucoma and
closed-angle glaucoma. Ophthalmologica 2004;218(5):344-349. [CrossRef] [PubMed]
6 Helbig H,
Schlotzer-Schrehardt U, Noske W, Kellner U, Foerster MH, Naumann GO.
Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger J Ophthalmol 1994;3(3):148-153. [PubMed]
7 Yu AL, Fuchshofer R, Kampik A,
Welge-Lussen U. Effects of oxidative stress in trabecular meshwork cells are
reduced by prostaglandin analogues. Invest
Ophthalmol Vis Sci 2008;49(11):4872-4880. [CrossRef] [PubMed]
8 Wong M, Huang P, Li W, Li Y, Zhang SS,
Zhang C. T-helper1/T-helper2 cytokine imbalance in the iris of patients with
glaucoma. PLoS One 2015;10(3):e0122184.
[CrossRef] [PMC free article] [PubMed]
9 Chua J, Vania M,
Cheung CM, Ang M, Chee SP, Yang H, Li J, Wong TT. Expression profile of
inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis 2012;18:431-438. [PMC free article] [PubMed]
11 Yang J, Patil RV, Yu H, Gordon M, Wax
MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol 2001;131(4):421-426. [CrossRef]
12 Hautala N, Glumoff V, Hautala T,
Vainio O. IL-2 may possess neuroprotective properties in glaucomatous optic
neuropathy. Acta Ophthalmol
2012;90(3):e246-247. [CrossRef] [PubMed]
13 Gramlich OW, Beck S, von Thun Und
Hohenstein-Blaul N, Boehm N, Ziegler A, Vetter JM, Pfeiffer N, Grus FH.
Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody
accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS One 2013;8(2):e57557. [CrossRef] [PMC free article] [PubMed]
15 Huang P, Zhang SS, Zhang C. The two
sides of cytokine signaling and glaucomatous optic neuropathy. J Ocul Biol Dis Infor 2009;2(2):78-83. [CrossRef] [PMC free article] [PubMed]
16 Kim M, Lee C, Payne R, Yue BY, Chang
JH, Ying H. Angiogenesis in glaucoma filtration surgery and neovascular
glaucoma-a review. Surv Ophthalmol 2015;60(6):524-535. [CrossRef] [PMC free article] [PubMed]
17 Yuan J, Guo Q, Qureshi AR, Anderstam
B, Eriksson M, Heimbürger O, Bárány P, Stenvinkel P, Lindholm B. Circulating
vascular endothelial growth factor (VEGF) and its soluble receptor 1(sVEGFR-1)
are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant 2013;28(9):2356-2363.
[CrossRef] [PubMed]
18 Morgan E, Varro R, Sepulveda H, Ember
JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D,
Gaur A. Cytometric bead array: a multiplexed assay platform with applications
in various areas of biology. Clin Immunol
2004;110(3):252-266. [CrossRef] [PubMed]
19 Ooi KG, Galatowicz G, Towler HM,
Lightman SL, Calder VL. Multiplex cytokine detection versus ELISA for aqueous
humor: IL-5, IL-10, and IFN-α profiles in uveitis. Invest Ophthalmol Vis Sci 2006;47(1): 272-277. [CrossRef] [PubMed]
20 Hou XR, Miao H, Tao Y, Li XX, Wong
IY. Expression of cytokines on the iris of patients with neovascular glaucoma. Acta Ophthalmol 2015; 93(2):e100-104. [CrossRef] [PubMed]
21 Agarwal R, Agarwal P. Glaucomatous
neurodegeneration: an eye on tumor necrosis factor-alpha. Indian J Ophthalmol 2012;60(4):255-261. [CrossRef] [PMC free article] [PubMed]
22 Yang Z, Quigley HA, Pease ME, Yang Y,
Qian J, Valenta D, Zack DJ. Changes in gene expression in experimental glaucoma
and optic nerve transection: the equilibrium between protective and detrimental
mechanisms. Invest Ophthalmol Vis Sci 2007;48(12): 5539-5548. [CrossRef] [PubMed]
23 Fuchs C, Forster V, Balse E, Sahel
JA, Picaud S, Tessier LH. Retinal-cell-conditioned medium prevents TNF-
alpha-induced apoptosis of purified ganglion cells. Invest Ophthalmol Vis Sci 2005;46(8):2983-2991. [CrossRef] [PubMed]
26 Dong N, Xu B, Chu L, Tang X. Study of
27 Aqueous humor cytokines in type 2 diabetic patients with or without macular
edema. PLoS One 2015;10(4):e0125329.
[CrossRef] [PMC free article] [PubMed]
27 Sakurada Y, Nakamura Y, Yoneyama S,
Mabuchi F, Gotoh T, Tateno Y, Sugiyama A, Kubota T, Iijima H. Aqueous humor
cytokine levels in patients with polypoidal choroidal vasculopathy and
neovascular age-related macular degeneration. Ophthalmic Res 2015;53(1):2-7. [CrossRef] [PubMed]
28 Huang W, Chen S, Gao X, Yang M, Zhang
J, Li X, Wang W, Zhou M, Zhang X, Zhang X. Inflammation-related cytokines of
aqueous humor in acute primary angle-closure eyes. Invest Ophthalmol Vis Sci 2014;55(2):1088-1094. [CrossRef] [PubMed]
29 Takai Y, Tanito M, Ohira A. Multiplex
cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma,
exfoliation glaucoma, and cataract. Invest
Ophthalmol Vis Sci 2012;53(1):241-247. [CrossRef] [PubMed]
30 Abrahamsson TR, Sandberg Abelius M,
Forsberg A, Björkstén B, Jenmalm MC. A Th1/Th2-associated chemokine imbalance
during infancy in children developing eczema, wheeze and sensitization. Clin Exp Allergy 2011;41(12):1729-1739.
[CrossRef] [PubMed]
33 Freedman J, Iserovich P.
Pro-inflammatory cytokines in glaucomatous aqueous and encysted Molteno implant
blebs and their relationship to pressure. Invest
Ophthalmol Vis Sci 2013;54(7):4851-4855. [CrossRef] [PubMed]