
·Opinion·Current Issue·
·Achieve·
·Search Articles· ·Online Submission· ·About IJO· PMC
Citation: Hadayer A, Jusufbegovic D, Schaal S. Retinal
detachment repair through multifocal intraocular lens - overcoming
visualization challenge of the peripheral retina. Int J Ophthalmol
2017;10(6):1008-1010
Retinal detachment repair through multifocal
intraocular lens- overcoming visualization challenge of the peripheral retina
Amir Hadayer, Denis Jusufbegovic, Shlomit Schaal
Department
of Ophthalmology and Visual Sciences, University of Louisville, Louisville,
Kentucky 40202, USA
Correspondence
to: Shlomit Schaal. Department of Ophthalmology and Visual Sciences,
University of Louisville, 301 E Muhammad Ali Blvd, Louisville, Kentucky 40202, USA.
s.schaal@louisville.edu
Received:
2016-01-21
Accepted: 2017-03-13
DOI:10.18240/ijo.2017.06.27
Citation: Hadayer A, Jusufbegovic D, Schaal S. Retinal
detachment repair through multifocal intraocular lens - overcoming
visualization challenge of the peripheral retina. Int J Ophthalmol
2017;10(6):1008-1010
INTRODUCTION
Sir
Nicholas Harold Lloyd Ridley has revolutionized the practice of ophthalmology
by performing the first intraocular lens (IOL) implantation in 1949[1]. His scientific achievement was acknowledged thirty
years later, which led to US Food and Drug Administration approval in 1981[2]. Although the basic principles of IOL implantation have
not changed since, many efforts have been invested in perfecting IOL design
during the past decades.
While
the natural crystalline lens can dynamically accommodate and actively change
its refractive power, the conventional IOL implants cannot, as their refractive
power is fixed. Thus contemporary IOL research has been focusing on regaining
accommodation after cataract surgery. Currently, accommodative and
refractive/diffractive IOL designs are commercially available to offer possible
independence from glasses[3].
In
brief, accommodative IOLs have the capability of changing their physical
properties and thus dynamically change their refractive power. Refractive and
diffractive IOLs, which share similar features, have several different focal
points. They simultaneously create several (2-3) images one on top of the
other, for the brain to choose from: infinity, reading distance and optionally
an intermediate distance[4]. Diffractive and
refractive IOLs are known for inducing significant image distortion, glare, and
loss of contrast sensitivity, especially in mesopic conditions, when the pupil
is dilated. Nevertheless, and despite these adverse effects, their use and
popularity have significantly increased in recent years[5].
Modern
pars plana vitrectomy (PPV) has also been constantly evolving since first
introduced by Machemer[6] in 1970. PPV allows
delicate and controlled evacuation of the vitreous gel and further
sophisticated manipulation of the retina. Being a delicate surgical procedure,
PPV requires perfect visualization of the vitreous and retina up to the ora
serrata and beyond. Modern PPV heavily relies on the patient’s own optical
system, including the IOL implant, for visualization. Thus, the
diffractive/refractive type IOLs pose a significant new visualization challenge
for retinal surgeons.
Few
reports have been published to date confirming visualization difficulties
during vitrectomy with multifocal IOLs, nevertheless no solutions have been
offered so far. Yoshino et al[7] and
Kawamura et al[8] reported visualization
difficulty during vitrectomy for epiretinal membrane (ERM) peeling and for
retinal detachment respectively, caused by diffractive IOLs. On the other hand,
Marques et al[9] reported normal
visualization during PPV with accommodative IOL. This paper is the first to
demonstrate and suggest a few practical solutions to improve visualization
during vitrectomy with multifocal IOLs.
SURGICAL TECHNIQUE
After
informed consent was obtained, a 3-port vitrectomy surgery for rhegmatogenous
retinal detachment repair was performed, using a wide field contact indirect
lens. The 27-gauge valved trocars (Alcon Laboratories, Inc., Fort Worth, TX,
USA) were inserted 3.5 mm posterior to the limbus inferotemporally (infusion
line port), superonasally and superotemporally. During the first stages of the
surgery, visualization of the posterior pole and periphery was only mildly
compromised by the diffractive IOL. The Placido Disc pattern of the diffractive
surface of the IOL slightly distorted the retinal image, but the retinal image
was reasonable, especially when viewed through the central zone or in between
the optical zones (Figure 1).

Figure
1 Retinal view through a multifocal IOL under fluid, using a wide-field contact
lens.
After
vitrectomy the retina was inspected and the culprit break was marked. Nevertheless,
after fluid-air exchange was performed, the funduscopic view became blurry to a
degree where the marked retinal tear could not be seen (Figure 2). A 30-gauge
needle was then used to coat both surfaces of the IOL with a thin layer of
viscoelastic material (HEALON® OVD, Abbott Medical Optics, Santa Ana, CA, USA),
injecting behind the IOL through pars plana and injecting anteriorly through
the anterior chamber, and the hand-held indirect contact lens was slightly
tilted. The surgeon’s view was hence vastly improved, allowing for safely
completing the surgery (Figure 3). The subretinal fluid was drained through the
retinal break using a soft-tip Charles Flute, and the retina completely
reattached. Air was then exchanged with 25% SF6 (sulfur hexafluoride),
the viscoelastic was removed from the anterior chamber using balanced salt
solution irrigation, and finally the trocars were removed.
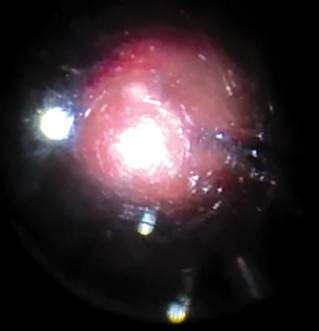
Figure
2 Initial retinal view through a multifocal IOL under air, using a wide-field
contact lens.
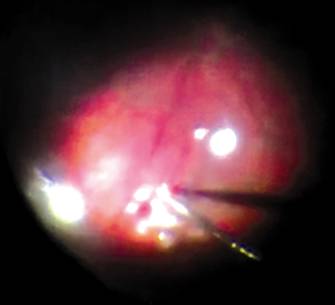
Figure
3 Improved retinal view through a multifocal IOL under air, using a wide-field
contact lens, after coating the IOL anteriorly and the posterior capsule
posteriorly with Healon, and slightly tilting the contact lens.
DISCUSSION
Modern
vitrectomy surgery requires excellent visualization of the vitreous and retina,
and relies upon the patient’s own optical system. Multifocal IOLs are optically
designed to trade image quality with glasses independence. Their optical design
reduces image quality and contrast sensitivity, and causes glare, all of which
worsen as the pupil dilates. Surgeon’s fundus view is closely correlated with
patient’s view, and therefore also becomes compromised in eyes with implanted
multifocal IOLs, more so as the pupil is pharmacologically dilated[10].
Visualization
during a standard PPV under air is more challenging compared to PPV under
water, because the refractive index difference between the IOL and gas is
higher relative to the refractive index difference between the IOL and liquid.
Under these conditions slight surface irregularities induce a greater optical
distortion of the image. Therefore, the poorest surgeon’s view is expected
during PPV under air with a multifocal lens and a dilated pupil.
The
physical structure of the multifocal IOLs dictates that the visualization
artifacts change in quality and severity depending on the target’s location and
light’s path to the surgeon’s viewing system. Thus, areas of interest can
rapidly seem to disappear and reappear in a different place or become abruptly
optically distorted as the light crosses different IOL optical zones. In that
aspect, macular surgery is different from retinal detachment repair surgery,
since in the former the surgeon can experience a more stable image and less
distortion as long as the light is tunneled through the central optical zone of
the IOL. In contrast, during retinal detachment surgery the entire optical
system is in a brisk constant change, which requires much more effort and
skills to maintain optimal visualization.
To
overcome these visualization challenges, fluid-air exchange should be deferred
until view-sensitive surgical stages have been completed. By tilting the
optical system, the image reflected from the interface surfaces may be steered
away from the surgeon’s viewing system. Shielded (beveled) illumination may be
used to block the light from directly scattering and reflecting into the
viewing system. Wide-field indirect viewing systems use condensing lenses and
the image obtained is less affected by media irregularities. By coating the IOL
with a thin layer of viscoelastic material, the refractive/diffractive effect
of the multifocal IOL is attenuated, as well as other surface irregularities
such as IOL scratches and posterior capsule irregularities. According to
Snell’s law of refraction, when light passes through refractive elements, the
degree of ray diversion is proportional to their refractive indices difference.
Thus, by coating the IOL with viscoelasticity, the surface irregularities of
the IOL (including the refractive/diffractive elements) have less optical
influence, and thus the fundus image improves. This same principle is naturally
prevalent in the eye where the corneal epithelium is coated with a thin mucin
layer. While most viscoelastic compounds may achieve this goal, the use of a
dispersive agent is preferred since these typically produce a smooth and even
coating. This technique is also useful to avoid fogging and condensations on
the IOL surface when operating under air[11-12].
This
paper discusses a few principles on ways to improve visualization through
multifocal IOLs during vitrectomy surgery. We routinely employ the discussed
techniques in our vitrectomy cases, such as the use of indirect contact
visualization system that is slightly tilted, and coating of the IOL with
viscoelasticity when multifocal IOLs and poor fundus view are present.
The
above described techniques should usually be sufficient to enable conventional
PPV. Nevertheless, in extreme cases, and when other media problems co-exist,
endoscopic vitrectomy, IOL removal, open-air vitrectomy or
keratoprosthesis-assisted vitrectomy may be indicated. As multifocal IOL
implants become general practice during cataract extraction, further research
is required to find solutions for improving image quality and visualization
during vitrectomy.
ACKNOWLEDGEMENTS
Authors’
Contributions: Dr. Schaal had full access to all of the data in the study and
takes responsibility for the integrity of the data and the accuracy of the data
analysis. All authors have had substantial contributions to design of the work,
or the acquisition, analysis, or interpretation of data for the work; and
drafting of the work and agreement to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Foundations:
Supported in part by an unrestricted grant from Research to
Prevent Blindness, Inc.; The American Physician Fellowship for Medicine in
Israel.
Conflicts
of Interest: Hadayer A, None; Jusufbegovic D, None; Schaal S,
None.
REFERENCES
1
Apple DJ, Sims J. Harold Ridley and the invention of the intraocular lens.
<ii>Surv Ophthalmo </ii>1996;40(4):279-292. [CrossRef]
2
Pandey SK, Apple DJ. Professor Peter Choyce: an early pioneer of intraocular
lenses and corneal/refractive surgery. <ii>Clin Exp Ophthalmol
</ii>2005;33(3):288-293. [CrossRef]
[PubMed]
<no>3 Calladine
D, Evans JR, Shah S, Leyland M. Multifocal versus monofocal intraocular lenses
after cataract extraction. <ii>Cochrane Database Syst Re
</ii>2012;(9):CD003169.</no>
4
Davison JA, Simpson MJ. History and development of the apodized diffractive
intraocular lens. <ii>J Cataract Refract Surg
</ii>2006;32(5):849-858. [CrossRef]
[PubMed]
5
Cillino S, Casuccio A, Di Pace F, Morreale R, Pillitteri F, Cillino G, Lodato
G. One-year outcomes with new-generation multifocal intraocular lenses.
<ii>Ophthalmology </ii>2008;115(9):1508-1516. [CrossRef]
[PubMed]
6
Machemer R. The development of pars plana vitrectomy: a personal account.
<ii>Graefes Arch Clin Exp Ophthalmol </ii>1995;233(8):453-468. [CrossRef]
7
Yoshino M, Inoue M, Kitamura N, Bissen-Miyajima H. Diffractive multifocal
intraocular lens interferes with intraoperative view. <ii>Clin Ophthalmol
</ii>2010;4:467-469. [PMC free article] [PubMed]
8
Kawamura R, Inoue M, Shinoda K, Bissen-Miyajima H. Intraoperative findings
during vitreous surgery after implantation of diffractive multifocal
intraocular lens. <ii>J Cataract Refract Surg
</ii>2008;34(6):1048-1049. [CrossRef]
[PubMed]
9
Marques EF, Ferreira TB, Castanheira-Dinis A. Visualization of the macula
during elective pars plana vitrectomy in the presence of a dual-optic
accommodating intraocular lens. <ii>J Cataract Refract Surg
</ii>2014;40(5):836-839. [CrossRef]
[PubMed]
10
Davison JA, Simpson MJ. History and development of the apodized diffractive
intraocular lens. <ii>J Cataract Refract Surg</ii>
2006;32(5):849-858. [CrossRef]
[PubMed]
11
Hainsworth DP, Chen SN, Cox TA, Jaffe GJ. Condensation on
polymethylmethacrylate, acrylic polymer, and silicone intraocular lenses after
fluid-air exchange in rabbits. <ii>Ophthalmology
</ii>1996;103(9):1410-1418. [CrossRef]
12
Ahmad BU, Shah GK, Hardten DR. Presbyopia-correcting intraocular lenses and
corneal refractive procedures: a review for retinal surgeons. <ii>Retina
</ii>2014;34(6):1046-1054. [CrossRef]
[PubMed]