
·Basic Research· Current
Issue IF in JCR CiteScore ·Submission· In Press Recent Accepted PMC RSS
Citation: Ahuja S. Possible role of sialylation of retinal protein glycans in the
regulation of electroretinogram response in mice. Int J Ophthalmol 2017;10(8):1217-1222
Possible role of sialylation of retinal protein
glycans in the regulation of electroretinogram response in mice
Satpal Ahuja
Department
of Ophthalmology, Biomedical Centre, Block 11, Klinikgatan 26, Institute of Clinical
Sciences, Lund University, Lund 221 84, Sweden
Correspondence
to: Satpal Ahuja. Department of Ophthalmology, Biomedical Centre,
Block 11, Klinikgatan 26, Institute of Clinical Sciences, Lund University, Lund
221 84, Sweden. sat_pal.ahuja@med.lu.se; satpal.ahuja@gmail.com
Received:
2016-08-25
Accepted: 2017-05-25
Abstract
AIM:
To evaluate if the nature, degree and extent of Siaα2-3-/Siaα2-6-sialylation
of retinal protein glycans plays a possible role in the development and
regulation of electroretinogram response (ERG) in mice.
METHODS: Proteins
extracted, from retinae of postnatal day 2 (PN2), PN7, and PN14 wild type (wt)
and retinal degeneration 1 (rd1) mice were quantified, labeled and used for
lectin-microarray profiling with immobilized lectins which recognize a wide
range of N-/O-glycans. Net fluorescence intensities of lectin-ligand complexes
were measured and images of fluorescent lectin-microarrays were acquired. From
the binding curves between each lectin and protein extracts from PN14 wt and
PN14 rd1 mice retinae, the protein concentration was selected to determine
optimum signal intensity for lectin-ligand binding. Mean±SEM values of proteins
and fluorescence-intensities of lectin-ligand-complexes between 45 lectins and
36 protein extracts from wt and rd1 mice retinae were compared for significance
of differences.
RESULTS: Comparison
of the progressive relative changes in the sialylated glycans of retinal
proteins from wt and rd1 mice showed that Siaα2-3Galβ1-4GlcNAc-glycans
(but not Siaα2-6-glycans)
were detectable and quantifiable from the retinal-proteins of PN7 and PN14 wt
and rd1 mice. Siaα2-3-sialylation
of retinal-protein Gal/α-linked-Gal-glycans
was significantly increased with age in PN7 and PN14 wt and less so in PN14 rd1
mice. Siaα2-3-/Siaα2-6-sialylation
of retinal-protein Gal/α-linked-Gal-glycans
was absent in PN2 wt and rd1 mice. Comparison of published ERG responses
of wt and rd1 mice retinae with degree of Siaα2-3-sialylation
of retinal-protein-glycans showed that PN2 wt and rd1 mice lack both the ERG
response and Siaα2-3-/Siaα2-6-sialylation
of retinal-protein Gal/α-linked-Gal-glycans;
rd1 mice with relatively lower Siaα2-3-sialylation
of retinal-protein Gal/α-linked-Gal-glycans
showed aberrant ERG response; and wt mice with significantly higher Siaα2-3-sialylation
of retinal-protein Gal/α-linked-Gal-glycans
showed normal ERG response.
CONCLUSION: Degree
of Siaα2-3-sialylation
of glycans possibly regulates ERG function in mice.
KEYWORDS: electroretinogram response; glycome; lectin microarray; mice
retinae; retinal development and degeneration
DOI:10.18240/ijo.2017.08.05
Citation: Ahuja S. Possible role of sialylation of retinal protein glycans in the
regulation of electroretinogram response in mice. Int J Ophthalmol 2017;10(8):1217-1222
INTRODUCTION
Rod
photoreceptor cGMP phosphodiesterase type 6 (PDE-6), an effector enzyme in the
retinal photo-transduction cascade, is activated by the active form of
G-protein transducin to hydrolyze cGMP. Resulting decrease in cGMP levels
closes cGMP-gated channels, transiently hyper-polarizes rod photoreceptors’
(PR) plasma membrane (PM) and decreases the release of neurotransmitter (NT)
glutamate at the PR synapse. Wild type (wt) mice show normal retinal
architecture and electroretinogram response (ERG). Retinal degeneration 1 (rd1)
mouse, an animal model of retinitis pigmentosa, is deficient in PDE-6 activity
due to a mutation in the β-subunit of PDE-6 gene. Resulting increase in cGMP
level opens cGMP-gated channels, activates Ca2+-ion channels,
prolongs release of glutamate and depolarizes PR membranes. Such PR degenerate
rapidly and show aberrant ERG response[1-3].
Repertoire of glycans, decorating PM proteins varies during tissue development
and degeneration[4-8]; influences
neuronal-signaling, angiogenesis and inflammation by binding to cis-/trans- Siglecs
and Galectins respectively which specifically bind to sialic acid (Sia) glycans
and galatans[9-12].
Nature,
Biosynthesis and Function of Retinal Glycans According to
the nature of linkage between glycans and amino acid residues of proteins,
mammalian cell glycans are classified as O-/N-linked oligosaccharides which are
linked respectively to hydroxyl group of Thr, Ser and amino group of Asn
residues of glycoproteins (GP) (Figure 1a). Membrane glycoprotein glycome shows
cell/tissue type specificity, structural diversity, and dynamic quantitative
changes during tissue development and degeneration. Diversity in the nature and
linkages of glycome saccharides generates glycoprotein heterogeneity; which
influences biological processes[13-17].
Sequence of saccharides in the glycome also encodes information for the
conformation and spatial arrangement of glycoproteins in the PM[5-7,10]. Glycans
displayed by glycoproteins modulate voltage-gated ion-channels, formation of
synaptic-junction and release of NTs[17-21].
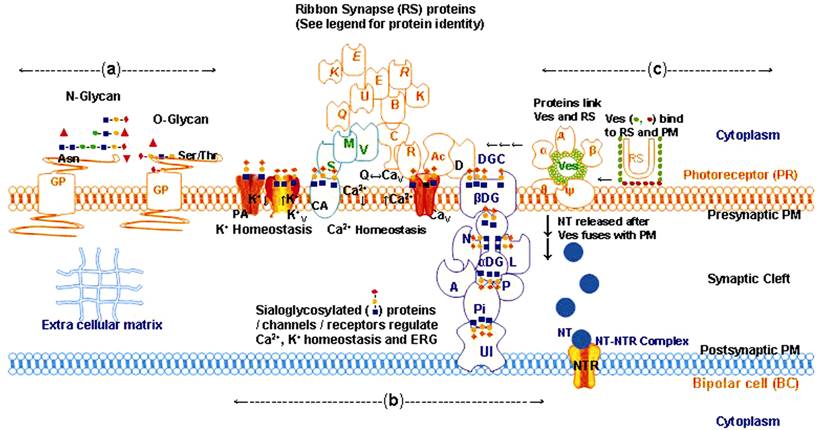
Figure 1 Sialylation of retinal protein glycans possibly establishes
synaptic junctions between PR- and bipolar-cells, and regulates retinal
integrity, retinal function and ERG
Asn: Asparagine; BC: Bipolar cell; DGC:
Dystrophin glycoprotein complex; ECM: Extracellular matrix; NTR:
Neurotransmitter receptor; PR: Photoreceptor; PM: Plasma membrane; Ser: Serine;
Thr: Threonine.
The
arrangement of different GP and their glycans as well as of ribbon synapse
(RS)/vesicles (Ves) in the PM, and of different families of proteins involved
in RS function given in Figure 1 are as follows: namely a) GP anchored in the PM
display Siaα2-3-sialylated (red-diamond, orange-circle, blue-square) N-glycans
and O-glycans, Fuc (Fucose, red-triangle); man (mannose, green-circle); Gal
(galactose, orange-circle); GalNAc (N-Acetyl galactosamine, orange-square);
GlcNAc (N-Acetyl glucosamine, blue-square); Sia (red-diamond). b) Sialylated
glycans displayed by GP Ca2+ATPase (CA) voltage-gated Ca2+ ion
channel (CaV) and α,β-dystroglycan (α,β-DG) connect with the proteins Ac, D, Q, E, B, E, K,
R, K, R, C, U, V, M, S, N, A, L, P, Pi and UI (see below for
identity of proteins, red, green, blue color outlines) so as to participate in
the generation of an ERG response. In absence of sialylated glycans the GP CA,
CaV and α,β DG are unable to maintain connectivity between
glycoprotein-protein complexes especially through Ac, D, R, Pi and UI proteins
which dampens the ERG response. Red, green and blue color outline represent
different groups of proteins; Synaptophysin (α); Synaptobrevin (β); Complexin
(д); SNAP25 synaptosome-associated 25 kDa protein (θ); Syntaxin (ψ); actin
(Ac); dystrophin (D); Ca2+ binding protein 4 (Q); Ribeye A (E);
bassoon (B); Ribeye B (E); piccolo (K); Rab3-interacting
molecules (R); Kif3a kinesin family member 3a protein (K);
RIM1 RIM2 Rab3-interacting molecules (R); CAST cytomatrix protein of the active
zone of RS (C); Munc13 (U); Veli3 (V); MPP4 membrane palmitoylated protein 4
(M); PSD95 post synaptic density protein (S); neurexin (N); agrin (A); laminin
(L); perlecan (P); pikachurin (Pi); unidentified (UI) protein; PA, Ca2+-activated
K+-channel; KV: Ca2+ activated voltage-gated K+
ion channel. Details of the identity, sialylation status and interactions
between these proteins are from different reference number[16,22-25]. c) Horse shoe shaped RS is
studded with lateral (olive-green-circle) and horizontal (mauve-circle) Ves
which are filled with NT (blue-circle). Fusion of Ves to RS and the latter to
pre-synaptic PM possibly involves proteins α, β, д, θ and ψ.
In
mammalian cells, the nature of linkage and type and level
of glucosylated-, galactosylated-, mannosylated-, fucosylated- and
sialylated-glycans displayed by GP are influenced by a balance in the
activities of the following pairs of enzymes namely
glucosyltransferase/glucosidase, galactosyltransferase/galactosidase, mannosyltransferase/mannosidase,
fucosyltransferase/fucosidase and sialyltransferase/sialidase, respectively. Sia,
a signaling molecule, terminally masks the penultimate α2-3Gal-, α2-3GalNAc-,
α2-6Gal- or α2-6GalNAc-glycan epitopes displayed by GP. Terminal Sia decorating
neuronal receptors is recognized by cis-/trans- Siglecs and provides
neuro-protection, modulates neural-differentiation and -integrity during tissue
development and degeneration[7,9-10]. Specific sialyltransferases link Siaα2,3- to β-D-Gal-glycans and
Siaα2,6- to β-D-Gal/β-D-GalNAc/β-D- GlcNAc-glycans. Rodent retina shows high activities of
sialyl-, fucosyl- and galactosyl-transferases and differentially expresses
α2,3-sialyltransferases/α2,6-sialyltransferases during retinal development and
degeneration. Decrease in the expression of sialyltransferases and/or increase
in the activity of sialidases limit the number of terminally linked Sia
residues leading to the unmasking of the penultimate
β-Gal/β-D-GalNAc/β-D-GlcNAc-glycans. Unmasked GalGlcNAc, α1,2-fucosylated, but
not the α1,3/4-fucosylated or α2,6-sialylated terminal glycans displayed by GP
(namely laminin, fibronectin, integrin, transferrin and lysosomal membrane
proteins) are recognized by cis-/trans- Galectins to influence
angiogenesis and inflammation[26-31].
However, significance of the sialylated glycans in retinal biology is unknown
and was therefore studied.
MATERIALS AND METHODS
Details of the ethical approval, general methodology for progressive and
relative quantification of the glycome by lectin microarray technique and for
statistical analyses are the same as given in Ahuja[17].
Briefly, retinae from postnatal day 2 (PN2), PN7, and PN14 wt and rd1 mice (6
replicates each, total number 36) were dissected. Retinal proteins were
extracted, quantified, labeled with Cy3 fluorescent dye, diluted (between 31.25
and 2000 ng·mL-1) with Probing Solution (GP BioSciences Ltd.,
Yokohama, Japan). Diluted protein extracts were used (in triplicates) for
lectin microarray profiling with 45 lectins immobilized on LecChip Ver 1.0 (GP
BioSciences Ltd.). Net fluorescence intensities of lectin-ligand complexes were
measured (in quadruplicate) and images of fluorescent lectin microarrays were
acquired by using the evanescent-field fluorescence scanner (GlycoStation
Reader 1200 GP BioSciences Ltd.). Results were analyzed after expanding the
dynamic range of this data by gain merging method using GlycoStation Tools Pro
Suite 1.5 (GP BioSciences Ltd.). From the binding curves between each of the 45
lectins and protein extracts (protein concentration between 31.25 and 2000
ng·mL-1) from PN14 wt and PN14 rd1 mice retinae, protein
concentration of 62.5 ng·mL-1 was selected to determine optimum
signal intensity for lectin-ligand binding. Mean±SEM values of proteins and
fluorescence intensities of lectin-ligand complexes between 45 lectins and 36
protein extracts from PN2, PN7 and PN14 wt and PN2, PN7 and PN14 rd1 mice
retinae[17] were compared for statistical
significance of differences. One way ANOVA and Fisher’s protected least
significant differences; post-hoc comparisons were made (StatView
Software, SAS, Chicago, IL, USA) and assigned significance as follows: P≥0.05 non-significant;
P<0.05 significant; P≤0.01 very significant, P≤0.001
highly significant. Forty five lectins used during this study specifically
react with a wide range of N-/O-glycans displayed by proteins. Nomenclature,
abbreviations, basic carbohydrate specificities and source of the lectins are
as given by Hirabayashi et al[8].
RESULTS
By using the lectin microarray technique dynamic and relative
quantitative changes in the glycans of retinal proteins[17]
were derived from the carbohydrate specificities of the lectins and levels of
lectin-ligand complexes. For the first time a comprehensive repository of
dynamic and quantitative global changes, in the nature and quantities of
glycans representing PN2, PN7 and PN14 wt and rd1 mice retinal proteins, was
prepared and published by Ahuja[17]. However, the
significance of these changes in retinal biology could not be incorporated in
this publication due to the large number and diversity of the glycans with
different types of linkages and the same is described now.
Because of the interactions between Sia (a signaling molecule) and cis-/trans-
Siglecs (receptor lectins for Sia) Sia influences patho-physiological
processes[32]. Therefore, sialylation status
(namely nature of linkages and extent of sialylation) of retinal glycans
representing six different patho-physiological states of mice was selected from
the repository of glycans referred above[17].
Changes in the sialylated glycan specificity of these lectin-ligand complexes
have now been compared with the published ERG status of wt and rd1 mice retinae[1,3] so as to determine the role of Sia
in the regulation of retinal function especially the ERG response[17].
Significance
of Siaα2-3-sialylation of Retinal Protein Glycans in
the Regulation of
Electroretinogram
Response From the analysis of the glycan specificity of lectin-ligand
complexes it was evident that the unmasked Gal/GalNAc epitopes lacking
Siaα2-3/Siaα2-6-sialylation were significantly increased (due to lower
sialylation or higher desialylation) specifically in retinal proteins of rd1
mice[17]. And content of Fucα1-6GlcNAc (core
Fuc)-, Fucα1-6GlcNAc- and Fucα1-6GlcNAc/Fucα1-3 (Galβ1-4) GlcNAc-glycans
detected by the lectins AOL LCA and AAL (respectively from Aspergillus
orizae, Lens culinaris and Aleuria aurantia) in retinal
proteins of PN2, PN7 and PN14 wt (P<0.05 to P≤0.001) and rd1 (NS
to P≤0.01) (except that by AAL) mice were significantly decreased with
age. The decrease was lower in rd1 retinal protein glycans (due to lower
sialylation or higher desialylation) as compared to those from wt mice.
Fucα1-2Galβ1- or GalNAcβ1-glycans recognized by the lectin TJA-II (from Tricosanthes
japonica) were detected only in rd1 retinal proteins especially those from
PN7 rd1 retinae. Content of GalNAc-, Galβ1-4GlcNAc- and
Galβ1-3GalNAc-glycans respectively detected by the lectins TxLC-I, RCA-120 and
PNA (respectively from Tulipa gesneriana, Ricinus communis and Arachis
hypogaea) formed a minor component but were specifically higher in PN7 rd1
retinal proteins. Galβ1-4GalNAc-, α-linked terminal GalNAc-,
Galβ1-3GalNAc/GalNAc-, and α-linked-Gal-glycans respectively detected by the
lectins DSA, HPA, Jacalin and GSL-IB4 (respectively from Datura stramonium,
Helix pomatia, Artocarpus integliforia and Griffonia simplicifolia)
formed bulk of the galactosylated glycans. Galactosylated glycans recognized by
the lectins DSA and HPA were decreased with age and the decrease was
significantly (P<0.05) higher in the retinal proteins from wt
mice (due to higher sialylation or lower desialylation) as compared to that in
rd1 mice retinal proteins. The higher level of such non-sialylated
glycans especially in the PN7 rd1 mice retinal proteins was apparently due to
lower sialyltransferase or higher sialidase activities.
Siaα2-3Galβ1-4GlcNAc-glycans recognized by the lectin ACG (from Agrocybe
cylindraacea) were detected in the retinal proteins both from PN7 and PN14
wt and rd1 mice but not in those from PN2 wt and rd1 mice. Siaα2-3Galβ1-4GlcNAc-glycan content of retinal proteins increased significantly
with age both in the wt and rd1 mice but the increase was higher (P≤0.01
to P≤0.001) in PN7 and PN14 wt (due to higher sialylation or lower
desialylation) as compared to that in the corresponding rd1 (NS to P≤0.01)
mice retinae. Out of the total sialylated glycans (recognized by the lectin
WGA, from Triticum aestivum), Siaα2-3Galβ1-4GlcNAc-glycan (recognized by the lectin ACG) constituted a relatively
small fraction of the glycome of proteins of both wt and rd1 mice retinae. The
higher level of sialylated glycans in wt mice retinal proteins is apparently
due to higher sialyltransferase or lower sialidase activities. Siaα2-6Gal/GalNAc-glycans recognized by the lectins SNA, SSA and TJA-1
(respectively from Sambucus nigra, Sambucus sieboldiana and Tricosanthes
japonica) and Siaα2-3Galβ1-4GlcNAc-
and Siaα2-3Galβ1-3 (Siaα2-6) GalNAc-glycan recognized by the
lectins MAL and MAH (from Maackia amurensis), respectively, were not
detected in any of the protein extracts from PN2, PN7 and PN14 wt and rd1 mice
retinae possibly due to low abundance of such sialylated proteins. PN2 wt and
rd1 mice retinae do not show ERG response. However, with increasing age, ERG
response develops normally in wt mice but is aberrant in rd1 mice retinae[1,3].
DISCUSSION
Due
to lack of appropriate technologies, the dynamically changing global profile,
nature and quantity of glycans and their significance in retinal biology during
retinal development and degeneration, has remained unexplored[23,33]. For such an evaluation lectin
microarray technology became available only after the year 2010. By using the
lectin microarray technique dynamic and relative quantitative changes in the
glycans of PN2, PN7 and PN14 wt and rd1 mice retinal proteins[17] were derived from the carbohydrate specificities of
the lectins and levels of lectin-ligand complexes. Changes in the sialylated
glycan specificity of these lectin-ligand complexes[17]
were compared with the published ERG status of wt and rd1 mice retinae[1,3] so as to determine the significance
of Sia in the development and regulation of retinal function especially the ERG
response. The possible role of Sialylated glycans associated with retinal
proteins in establishing/regulating ERG function has now been explained here
with respect to mice retinae.
Conventional
synapse and RS are two basic classes of synaptic-junctions in mammalian
retinae. At the conventional synapse between amacrine cells in the inner
plexiform layer (IPL) and ganglion cells in the ganglion cell layer, NT release
is triggered by brief bursts of action potential. At the RS between PR in the
outer plexiform layer and bipolar cells (BC) in the IPL, NT is released
continuously and the action potential change is gradual[22,28,34].
Significance
of Retinal Protein Glycans in the Regulation of Electroretinogram Response Electrophysiological
function of PR involves fusion of pre-/post-synaptic PM termini with horse shoe
shaped sheets of excitatory RS studded with glutamate filled lateral and
horizontal Ves (Figure 1c). Through sialylated glycans groups of proteins/GP in the RS connect
PR cytoskeleton with the extracellular matrix leading to the ERG response
(Figure 1b, 1c).
The
proteins labeled as α, β, д, θ and ψ (Figure 1c), attach lateral Ves to the RS;
and in a Ca2+-ion dependent manner move Ves to horizontal position
for fusion with the presynaptic PM of PR. Fusion of Ves to the pre-synaptic PM
and release of glutamate is regulated by Ca2+-ion homeostasis which
is achieved by modulating the activities of Ca2+-ATPase (CA) and
voltage-gated Ca2+-ion channel (CaV) (Figure 1b).
Glutamate is then released for binding to the neurotransmitter receptor (NTR)
anchored in post-synaptic PM of BC.
Through
sialylated glycans, GP CA, voltage-gated Ca2+-ion channel, α,β-DG of dystrophin glycoprotein complex (DGC), Ac, Ca2+-binding
protein 4 (Q) and dystrophin (D) interact with the RS proteins E, B, E,
K, R, K, R, C, U, V, M, S, N, A, L, P, UI and Pi (for identity of
the different groups of proteins see legends to Figure 1, red, green, blue
outlines). Sialylated glycans displayed by a number of these proteins possibly
form a bridge between PR and BC and establish cytoskeletal continuity[5-6] for the maintenance of
retinal-structure[16,22],
-function[12,23,28]
and ERG response as described in different references[24-25,34-36].
Significance
of the Degree of Sialylation of Retinal Protein Glycans in the Development and
Regulation of Electroretinogram Response
Lower degree of sialylation with Siaα2-3-/Siaα2-6- and higher
proportion of unmasked galactosylated/fucosylated glycans displayed by rd1 mice
retinal proteins suggests decreased sialyltransferase activity and/or increased
sialidase activity in rd1 mice retinae. Degenerating PR in rd1 mice retinae
have been shown to up-regulate α-Klotho, an anti-aging protein[37]. α-Klotho has homology with sialidase/glycosidase for
specific removal of Siaα2-6-residues to unmask and display GalGlcNAc-glycan
(recognized by cis-/trans- Galectins) of CaV and
voltage-gated K+-ion channel (KV) in kidneys[38]. An imbalance in the activities of sialyltransferase
and sialidase decreases sialylation of Gal/GalNAc-glycans of α-dystroglycan (α-DG)[13] which becomes unable to
maintain connectivity with the proteins namely pikachurin (Pi), RIM2 (R), Ac,
dystrophin (D) and an UI protein involved in the development of ERG response in
mice[5-6,18].
ERG aberration in rd1 mice[1,3] could
thus be attributed to the decrease in Siaα2-3-/Siaα2-6-sialylation of glycans
by retinal sialidase activity. As reviewed above, GP CaV, CA, α,β-DG
and KV have approximately 40%
of their mass as glycans, of which ≥45% consists of Sia residues. And
sialylated glycan based interactions between CaV, KV and
CA, Pi, α,β-DG and some UI protein
apparently regulate the ERG response in the wt and rd1
mice retinae.
It
is concluded that: PN2 wt and rd1 mice lack Siaα2-3-/Siaα2-6-sialylation of
retinal-protein Gal/α-linked-Gal-glycans and ERG response; rd1 mice with
relatively lower Siaα2-3-sialylation of retinal-protein Gal/α-linked-Gal-glycans
showed aberrant ERG response; and wt mice with significantly higher
Siaα2-3-sialylation of retinal-protein Gal/α-linked-Gal-glycans showed normal
ERG response. Targeting of Siglecs with Sia decorated nanoparticles has been
shown to abrogate inflammation[32] and similar
approach may be adopted for sialylation of desialylated RS proteins in mice
retinae showing aberrated ERG function. These results suggest that extent of
Siaα2-3-sialylation of retinal-protein Gal/α-linked-Gal-glycans possibly
influence the development and maintenance of the ERG responses in mice.
Overall,
the above findings suggest that lack or deficiency of Siaα2-3-sialylated
Gal/α-linked-Gal-glycans and degree of Siaα2-3-sialylation of retinal protein
glycans appears to have a possible regulatory role in the development and
maintenance of the ERG function in mice retinae. As retinal proteins deficient
in Sia show lack of protein-protein interaction[7,9-10,29], so
sialylation pattern of retinal proteins serves as a hallmark of protein-protein
interaction for ERG function, retinal integrity and health. These observations
along with those in Ahuja[17] on retinal glycan
profiles could open new avenues for diagnostic and therapeutic use in
neurodegenerative diseases. By using TALENs or CRISPR-Cas9 programmable DNA
editing gene therapy technologies, Li et al[39]
restored dystrophin in iPSCs of Duchenne Muscular Dystrophy patients having a
mutated dystrophin. However, dystrophin is a glycoprotein, also present in the
mouse retina (Figure 1b) and possibly in other mammals as well. Restoration of
dystrophin protein or other such proteins, without consideration for the extent
of sialylation/glycosylation may not restore the function of glycosylated
proteins consequently the efficacy of the gene editing therapy.
ACKNOWLEDGEMENTS
The
author thanks Kyoko Yokota, Ryoko Sawada and Masao Yamada PhD, Chief Scientific
Officer, at GP BioSciences Ltd., Yokohama, Japan, for lectin microarray analyses;
Birgitta Klefbohm for collecting retinae; Poonam AhujaJensen MD, PhD and Sanjay
Ahuja MD, PhD for statistical analyses; Sten Andréasson MD, PhD, Prof and Head,
for infrastructural facilities; Per Ekström PhD for providing mice; and
Motifolio Inc., USA for providing Power Point Tool Kit which was used to
generate Figure 1.
Foundations: Supportted
by Ögonfonden Synfrämjande Forskning, Stöd Ögonforskningen, Umeå (Sweden);
Stiftelsen Kronprinsessan Margaretas Arbetsnämnd för synskadade (KMA, Sweden);
and Stiftelsen för synskadade i.f.d Malmöhus Län, Malmö (Sweden).
Conflicts of Interest: Ahuja S, None.
REFERENCES
1 Delyfer
MN, Forster V, Neveux N, Picaud S, Léveillard T, Sahel JA. Evidence for
glutamate-mediated excitotoxic mechanisms during photoreceptor degeneration in
the rd1 mouse retina. Mol Vis 2005;11: 688-696. [PubMed]
2 Ionita MA,
Pittler SJ. Focus on molecules: rod cGMP phosphodiesterase type 6. Exp Eye Res
2007;84(1):1-2. [CrossRef] [PubMed]
3 Gibson R,
Fletcher EL, Vingrys AJ, Zhu Y, Vessey KA, Kalloniatis M. Functional and
neurochemical development in the normal and degenerating mouse retina. J Comp
Neurol 2013;521(6):1251-1267. [CrossRef] [PubMed]
4 Leppänen
A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity
to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on
surface-bound extended glycans. J Biol Chem 2005;280(7):5549-5562. [CrossRef] [PubMed]
5 Ednie AR,
Bennett ES. Modulation of voltage-gated ion channels by sialylation. Compar
Physiol 2012;2(2):1269-1301. [CrossRef]
6 Omori Y, Araki
F, Chaya T, Kajimura N, Irie S, Terada K, Muranishi Y, Tsujii T, Ueno S, Koyasu
T, Tamaki Y, Kondo M, Amano S, Furukaioa T. Presynaptic dystroglycan-pikachurin
complex regulates the proper synaptic connection between retinal photoreceptor
and bipolar cells. J Neurosci 2012;32(18):6126-6137. [CrossRef] [PubMed]
7 Wielgat P,
Braszko JJ. The participation of sialic acids in microglia-neuron interactions.
Cell Immunol 2012;273(1):17-22. [CrossRef] [PubMed]
8
Hirabayashi J, Yamada M, Kuno A, Tateno H. Lectin microarrays: concept,
principle and applications. Chem Soc Rev 2013;42(10):4443-4458. [CrossRef] [PubMed]
9 Croci DO,
Cerliani JP, Pinto NA, Morosi LG, Rabinovich GA. Regulatory role of glycans in
the control of hypoxia-driven angiogenesis and sensitivity to anti-angiogenic
treatment. Glycobiology 2014;24(12): 1283-1290. [CrossRef] [PubMed]
10
Linnartz-Gerlach B, Kopatz J, Neumann H. Siglec functions of microglia. Glycobiology
2014;24(9):794-799. [CrossRef] [PubMed]
11 Markowska
AI, Cao Z, Panjwani N. Glycobiology of ocular angiogenesis. Glycobiology 2014;24(12):1275-1282.
[CrossRef] [PMC free article] [PubMed]
12 Scott H,
Panin VM. The role of protein N-glycosylation in neural transmission. Glycobiology
2014;24(5):407-417. [CrossRef] [PMC free article] [PubMed]
13 Chiba A,
Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A,
Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral
nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide
in the binding of alpha-dystroglycan with laminin. J Biol Chem
1997;272(4):2156-2162. [CrossRef]
14 McDearmon
EL, Combs AC, Ervasti JM. Core 1 glycans on alpha-dystroglycan mediate
laminin-induced acetylcholine receptor clustering but not laminin binding. J
Biol Chem 2003;278(45):44868-44873. [CrossRef] [PubMed]
15 Johnson
D, Montpetit ML, Stocker PJ, Bennett ES. The sialic acid component of the beta1
subunit modulates voltage-gated sodium channel function. J Biol Chem 2004;279(43):44303-44310. [CrossRef] [PubMed]
16 Sato S,
Omori Y, Katoh K, Kondo M, Kanagawa M, Miyata K, Funabiki K, Koyasu T, Kajimura
N, Miyoshi T, Swai H, Kobayashi K, Tani A, Toda T, Usukura J, Tano Y, Fujikado
T, Furukawa T. Pikachurin, a dystroglycan ligand, is essential for
photoreceptor ribbon synapse formation. Nat Neurosci 2008;11(8):923-931. [CrossRef] [PubMed]
17 Ahuja S.
Lectin microarray profiling and relative quantification of glycome associated
with proteins of neonatal wt and rd1 mice retinae. Invest Ophthalmol Vis Sci
2013;54(5):3272-3280. [CrossRef] [PubMed]
18 Schwetz
TA, Norring SA, Ednie AR, Bennett ES. Sialic acids attached to O-glycans
modulate voltage-gated potassium channel gating. J Biol Chem
2011;286(6):4123-4132. [CrossRef] [PMC free article] [PubMed]
19 Dobson
CM, Hempel SJ, Stalnaker SH, Stuart R, Wells L. O-Mannosylation and human
disease. Cell Mol Life Sci 2013;70(16): 2849-2857. [CrossRef] [PMC free article] [PubMed]
20 Smith BJ,
Tremblay F, Côté PD. Voltage-gated sodium channels contribute to the b-wave of
the rodent electroretinogram by mediating input to rod bipolar cell GABA(c)
receptors. Exp Eye Res 2013;116: 279-290. [CrossRef] [PubMed]
21 Hall MK,
Weidner DA, Bernetski CJ, Schwalbe RA. N-Linked glycan site occupancy impacts
the distribution of a potassium channel in the cell body and outgrowths of
neuronal-derived cells. Biochim Biophys Acta 2014;1840(1):595-604. [CrossRef] [PubMed]
22 Schmitz F.
The making of synaptic ribbons: how they are built and what they do. Neuroscientist
2009;15(6):611-624. [CrossRef] [PubMed]
23 Kleene R,
Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci 2004;5(3):195-208.
[CrossRef] [PubMed]
24 Morgans
CW. Presynaptic proteins of ribbon synapses in the retina. Microsc Res Tech 2000;50(2):141-150.
[CrossRef]
25 Mercer
AJ, Thoreson WB. The dynamic architecture of photoreceptor ribbon synapses:
cytoskeletal, extracellular matrix, and intramembrane proteins. Vis Neurosci
2011;28(6):453-471. [CrossRef] [PMC free article] [PubMed]
26 Unoki K,
Uehara F, Muramatsu T. Distribution of glycosyltransferase in bovine eyes. Ophthalmic
Res 1990;22(6):342-350. [CrossRef]
27 Uehara F,
Ozawa M, Sameshima M, et al. Differential expression of mRNA for alpha
2,3-sialyltransferase during development of rat retina. Jpn J Ophthalmol
1995;39(3):248-253. [PubMed]
28
Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal
ribbon synapses. Prog Retin Eye Res 2005;24(6):682-720. [CrossRef] [PMC free article] [PubMed]
29 Schauer
R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin
Struct Biol 2009;19(5):507-514. [CrossRef] [PubMed]
30 Miyagi T,
Yamaguchi K. Mammalian sialidases: physiological and pathological roles in
cellular functions. Glycobiology 2012;22(7):880-896. [CrossRef] [PubMed]
31 Inafuku
S, Noda K, Amano M, Ohashi T, Yoshizawa C, Saito W, Murata M, Kanda A,
Nishimura S, Ishida S. Alteration of N-glycan profiles in diabetic retinopathy.
Invest Ophthalmol Vis Sci 2015;56(9):5316-5322. [CrossRef] [PubMed]
32 Spence S,
Greene MK, Fay F, Hams E, Saunders SP, Hamid U, Fitzgerald M, Beck J, Bains BK,
Smyth P, Themistau E, Small DH, Schmid D, O’Kane CM, Fitzgerald DC, Abdelghany
SM, Johnston JA, Fallon PG, Burrows JF, McAuley DF, Kissenpfennig A, Scott CJ.
Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates
inflammation. Sci Transl Med 2015;7(303):303ra140. [CrossRef] [PubMed]
33 Dani N,
Broadie K. Glycosylated synaptomatrix regulation of trans-synaptic signaling.
Dev Neurobiol 2012;72(1):2-21. [CrossRef] [PMC free article] [PubMed]
34 Schmitz
F, Drenckhahn D. Localization of dystrophin and beta-dystroglycan in bovine
retinal photoreceptor processes extending into the postsynaptic dendritic
complex. Histochem Cell Biol 1997;108(3):249-255. [CrossRef] [PubMed]
35 Zabouri
N, Haverkamp S. Calcium channel-dependent molecular maturation of photoreceptor
synapses. PLoS One 2013;8(5):e63853. [CrossRef] [PMC free article] [PubMed]
36 Schmitz
F. Presynaptic [Ca2+] and GCAPs: aspects on the structure and function of
photoreceptor ribbon synapses. Front Mol Neurosci 2014;7:3. [CrossRef] [PMC free article] [PubMed]
37 Farinelli
P, Arango-Gonzalez B, Völkl J, Alesutan I, Lang F, Zrenner E, Paquet-Durand F,
Ekström PA. Retinitis pigmentosa: over-expression of anti-ageing protein Klotho
in degenerating photoreceptors. J Neurochem 2013;127(6):868-879. [CrossRef] [PubMed]
38 Cha SK,
Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary
potassium channel and renal K (+) excretion by Klotho. Mol Pharmacol
2009;76(1):38-46. [CrossRef] [PMC free article] [PubMed]
39 Li HL,
Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T, Tanaka M, Amano N,
Watanabe A, Sakurai H, Yamamoto T, Yamanaka S, Hotta A. Precise correction of
the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent
stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports 2015;4(1):143-154. [CrossRef] [PMC free article] [PubMed]