IF in JCR CiteScore
Rank About IJO Current
Issue Featured Articles Articles In Press Recent Accepted
 International Journal
of Ophthalmology
International Journal
of Ophthalmology
2017; 10(9): 1385-1391
·Clinical Research·
Influence of corneal power on circumpapillary
retinal nerve fiber layer and optic nerve head measurements by spectral-domain
optical coherence tomography
Kazunori Hirasawa1, Nobuyuki Shoji2
1Department of Orthoptics and Visual
Science, School of Allied Health Sciences, Kitasato University, Sagamihara,
Kanagawa 252-0373, Japan
2Department of Ophthalmology, School of
medicine, Kitasato University, Sagamihara, Kanagawa 252-0373, Japan
Correspondence to: Kazunori Hirasawa. Department of Orthoptics and Visual
Science, School of Allied Health Sciences, Kitasato University, 1-15-1
Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan. hirasawa@kitasato-u.ac.jp
Received: 2017-02-24
Accepted: 2017-04-24
Abstract
Aim: To evaluate the influence of
corneal power on circumpapillary retinal nerve fiber layer (cpRNFL) and optic
nerve head (ONH) measurements by spectral-domain optical coherence tomography
(SD-OCT).
Methods: Twenty-five eyes
of 25 healthy participants (mean age 23.6±3.6y) were imaged by SD-OCT using
horizontal raster scans. Disposable soft contact lenses of different powers
(from −11 to +5 diopters including 0 diopter) were worn to induce 2-diopter
changes in corneal power. Differences in the cpRNFL and ONH measurements per
diopter of change in corneal power were analyzed.
Results: As corneal power
increased by 1 diopter, total and quadrant cpRNFL thicknesses, except for the
nasal sector, decreased by −0.19 to −0.32 μm
(P<0.01). Furthermore, the disc, cup, and rim areas decreased by −0.017,
−0.007, and −0.015 mm2, respectively (P<0.001); the cup
and rim volumes decreased by −0.0013 and −0.006 mm3, respectively (P<0.01);
and the vertical and horizontal disc diameters decreased by −0.006 and −0.007
mm, respectively (P<0.001).
Conclusion: For more precise
OCT imaging, the ocular magnification should be corrected by considering both
the axial length and corneal power. However, the effect of corneal power
changes on cpRNFL thickness and ONH topography are small when compare with
those of the axial length.
Keywords: optical coherence tomography; ocular
magnification; corneal power; circumpapillary retinal nerve fiber layer; optic
nerve head
Citation: Hirasawa K, Shoji N. Influence of corneal power on circumpapillary
retinal nerve fiber layer and optic nerve head measurements by spectral-domain
optical coherence tomography. Int J Ophthalmol 2017;10(9):1385-1391
Introduction
Spectral-domain optical coherence tomography (SD-OCT) enables the
detection of slight structural changes before visual field deterioration in
early glaucoma[1-14]. Such changes
are difficult to detect by traditional ophthalmoscopy or fundus photography.
However, measurements of structures such as the circumpapillary retinal nerve
fiber layer (cpRNFL) and optic nerve head (ONH) are influenced by factors
including axial length and high myopia independently of the degree of
glaucomatous change[13,15-27]. Therefore, these measurements should be corrected
according to the individual’s ocular magnification for accuracy.
Traditionally, Littmann’s[28] formula and
Bennett et al’s[29] modification are used
to correct for ocular magnification, as follows: t=p×q×s,
where t is the actual fundus dimension, p is the
magnification factor of the camera of the imaging system, q is the
magnification factor of the individual eye, and s is the value obtained
from the imaging device. Factor p is a constant in a telecentric system.
Factor q can be determined by the following formula[29]:
q=0.01306×(axial length −1.82).
Nevertheless, these formulas do not consider the optical properties of
the anterior segment, particularly the corneal power, because the position of
the second principal point is assumed constant. Researchers have investigated
the influence of corneal power on cpRNFL measurements by SD-OCT[30-32], but their findings are not
consistent. In addition, previous studies did not analyze the effect on ONH
measurements. In this study, we evaluated the influence of corneal power on
cpRNFL and ONH measurements by SD-OCT.
Subjects and Methods
This cross sectional study followed the tenets of the Declaration of
Helsinki. Written informed consent was obtained from each participant after
approval was received from the Ethics Committee of Kitasato University School
of Allied Health Science (No.2015-07). UMIN clinical trials registry
(http://www.umin.ac.jp/) under unique trial number UMIN000016698 (date of
registration: 03/03/2015).
Twenty-five healthy participants (mean age 23.6±3.6y, 3 males) underwent
comprehensive ophthalmic examinations, including noncycloplegic refraction
testing, visual acuity testing at 5 m using a Landolt ring chart, intraocular
pressure and axial length measurements, and slit-lamp and fundus examinations,
by a glaucoma specialist (Shoji N). For each participant, the eye with a
corrected visual acuity of 20/20 or better, intraocular pressure of 21 mm Hg or
lower, and more normal optic disc appearance was included in the study. If both
eyes met these inclusion criteria, the eye with lower astigmatism was included.
The cpRNFL thickness and ONH topography were measured by an SD-OCT
system (3D OCT-2000, version 8.1.1; Topcon, Tokyo, Japan) using the 3D optic
disc horizontal raster scan mode with a 512×128 scan resolution and 6 mm2
scan area. This device operates at a speed of 50 000 A-scans per second and has
a depth and lateral resolution of 6 μm and 20 μm or less, respectively. It requires a pupil size of 2.5 mm or larger
for imaging. Although the device can correct for ocular magnification on the
basis of Littmann’s[28] formula ocular
magnification was not corrected in this study.
A single expert examiner (Hirasawa K) performed all of the measurements
in the selected eyes without cycloplegia. The participants wore 10 differently
powered (from -11 to +5 diopters including plano) disposable soft contact
lenses (1-day Acuvue, Johnson & Johnson Vision Care, Inc., New Brunswick,
NJ, USA) in random order to change the corneal power, which was measured with
an auto kerato-refractometer (KR-8100PA, Topcon) before SD-OCT. When the signal
strength was unacceptable by over 40 at each contact lens power or when B-scan
line images were absent or deviated because of movement, the imaging was
repeated up to twice for each imaging. The following parameters were evaluated:
total and quadrant cpRNFL thicknesses, centered on the optic disc; disc, cup,
and rim areas; cup and rim volumes; vertical and horizontal disc diameters; and
image quality.
Statistical Analysis All data were analyzed using R software
(http://www.R-project.org) and G*Power3 version 3.1.7[33-34]. The effect size, α error,
power (1-β error), and nonsphericity correction were 0.25 (middle), 0.05, 0.95,
and 0.12, respectively, and the required sample size was 11 participants for 10
repeated measurements[35]. Using three sets of
measurements obtained with plano contact lenses, the repeatability was
calculated by the Bland and Altman method[36-37] as 2.77×Sw. Sw is the within-subject standard
deviation and formula is as follows:
Within subject standard deviation (Sw)= ![]()
Where ![]() is the standard
deviation of measurements on each subject, where n is the number of
participants. Intraclass correlation coefficients were also calculated. When the
confidence limit on either side of the estimate of Sw was set to 0.20, the
required sample size was 24 eyes.
is the standard
deviation of measurements on each subject, where n is the number of
participants. Intraclass correlation coefficients were also calculated. When the
confidence limit on either side of the estimate of Sw was set to 0.20, the
required sample size was 24 eyes.
The first set of measurements were obtained with plano contact lenses,
and data collected without a contact lens were compared by the paired t-test
to analyze the effect of contact lens wearing on cpRNFL and ONH measurements.
Differences of cpRNFL thickness and ONH parameter with different powers of
contact lenses were analyzed by repeated-measures analysis of variance.
Results
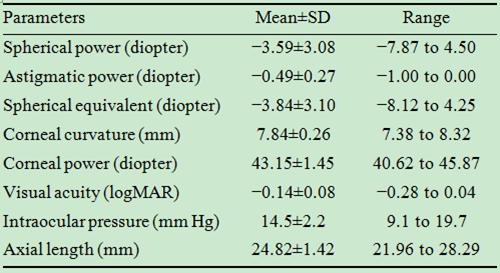
In this study, 15 right and 10 left eyes were imaged. Table 1 shows
their initial optical characteristics.
Table 1 Ocular characteristics of the participants
Contact lens wearing did not significantly affect the cpRNFL and ONH
measurements (Table 2) or their repeatability (Table 3).
Table 2 Measurements of cpRNFL thickness and ONH topography with plano
soft contact lens
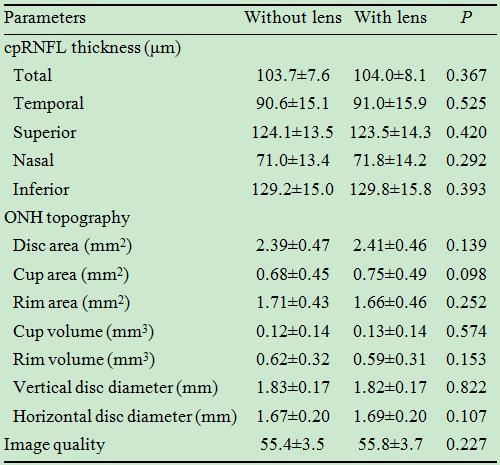
cpRNFL: Circumpapillary retinal nerve fiber layer; ONH: optic nerve head. Data
represent mean±SD, Sw, and 2.77×Sw.
Table 3 Repeatability of the measurements with plano soft contact lenses
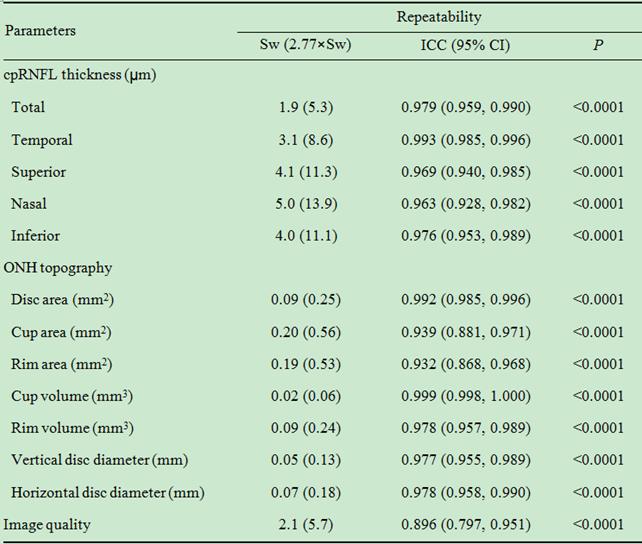
cpRNFL: Circumpapillary retinal nerve fiber layer; ONH: Optic nerve head; Sw:
Within-subject standard deviation; ICC: Intraclass correlation; CI: Confidence
interval.
As shown in Table 4, the measured cpRNFL thickness in every region
except for the nasal sector, ONH parameters, and image quality significantly
differed with varying contact lens powers (repeated-measures analysis of
variance, P<0.05). The changes in total cpRNFL thickness with
2-diopter induced increases in corneal power are depicted in Figure 1.
Table 4 Changes in the measurements with increasing soft contact lens
power
mean±SD
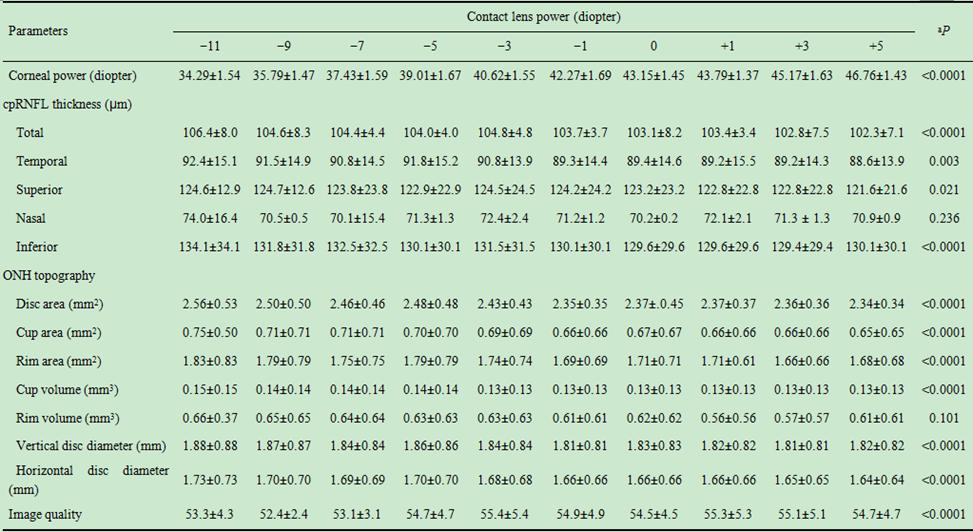
cpRNFL: Circumpapillary retinal nerve fiber layer; ONH: Optic nerve head. aP<0.05
is statistically significant by repeated-measure analysis of variance.
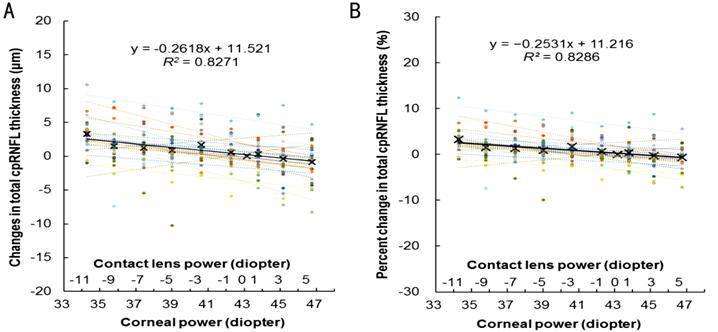
Figure 1 Actual (A) and percent (B) changes in total cpRNFL thickness
induced by increasing corneal power using soft contact lenses.
The different colored dots and their approximating lines indicate data
from individual participants. The crosses and solid line indicate the mean data
of all the participants.
Table 5 shows that the total cpRNFL thickness significantly decreased by
−0.26 μm (−0.25%, P<0.001) and the quadrant cpRNFL thickness, with
the exception of the nasal sector, significantly decreased by −0.19 to −0.32 μm (−0.17% to −0.25%, all P<0.007) as the corneal power
increased by 1 diopter. All ONH measurements also significantly decreased with
the 1-diopter-induced increases in corneal power (P<0.001). Only the
image quality increased (0.2 or 0.36% per diopter) with increasing corneal
power (P=0.007).
Table 5 Slope values of actual and percent changes in the measurements
per diopter increase in corneal power
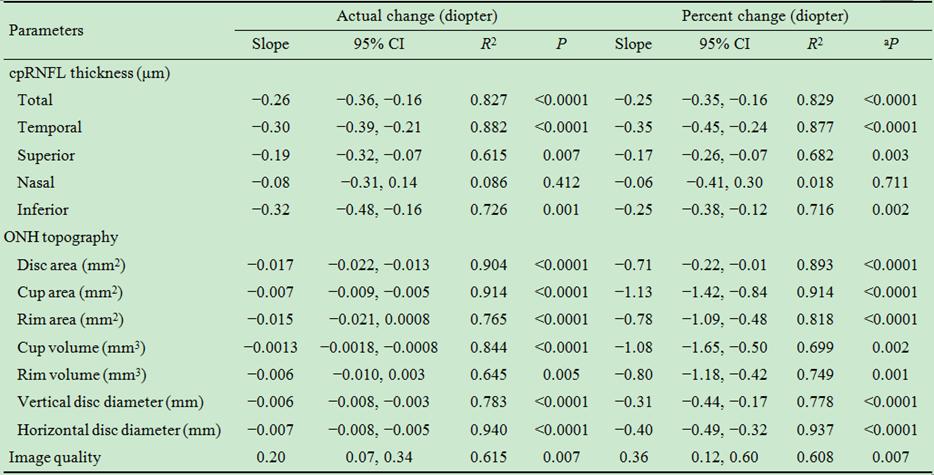
cpRNFL: Circumpapillary retinal nerve fiber layer; ONH: Optic nerve head; CI:
Confidence interval. aP<0.05 is statistically significant
by simple linear regression analysis.
Discussion
This study demonstrated good repeatability of the measurements with and
without a contact lens. Therefore, contact lens wearing does not introduce bias
in SD-OCT imaging. However, image quality reduces with induced decreases in
corneal power, in turn affecting assessment of cpRNFL thickness[38-39]. The current data might include
bias where image quality is concerned.
The total and quadrant cpRNFL thicknesses, except for nasal region,
showed up to 0.3 μm decreases (−0.4%), and ONH area
measurements were reduced up to 1.1% per diopter induced increase in corneal
power. One study showed that the total cpRNFL thickness measured by time-domain
OCT does not significantly differ with varying corneal power[32],
whereas another study demonstrated that cpRNFL thickness measured by SD-OCT
decreases by approximately 0.5 μm (−0.5%) per diopter induced increase in
corneal power[30-31]. Positional
variation of the second principal point due to changes in corneal power would
affect cpRNFL and ONH measurements.
In Littmann’s formula modified by Bennett et al[29], the second principal point is assumed to be located
at 1.82 mm from the corneal surface based on Bennett and Rabbetts’ schematic
eye[40]. However, its position moves backward and
forward when the corneal power becomes steeper and flatter, respectively,
because the calculation is based on the principal point of the crystalline
lens, corneal power, and total ocular power, as follows[40]:
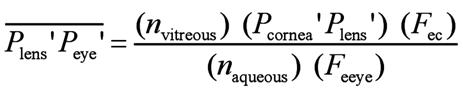
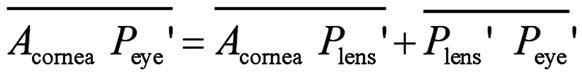
Where Plens' is the second principal plane of the
crystalline lens, Peye' is the second principal plane of the
eye, 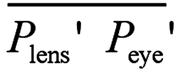 is the distance from the second principal
plane of the crystalline lens to the second principal plane of the eye, nvitreous
is the refractive index of the vitreous body, Pcornes' Plens'
is the distance from the second principal plane of the cornea to the second
principal plane of the crystalline lens, Fec is the
equivalent power of the cornea, naqueous is the refractive
index of the aqueous humor, Feye
is the equivalent power of the eye, Acornea is the anterior
surface of the cornea, and
is the distance from the second principal
plane of the crystalline lens to the second principal plane of the eye, nvitreous
is the refractive index of the vitreous body, Pcornes' Plens'
is the distance from the second principal plane of the cornea to the second
principal plane of the crystalline lens, Fec is the
equivalent power of the cornea, naqueous is the refractive
index of the aqueous humor, Feye
is the equivalent power of the eye, Acornea is the anterior
surface of the cornea, and 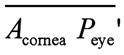 is the distance from the anterior surface
of the cornea to the second principal plane of the eye (second principal point).
By substituting the variation value of corneal power in the current study and
other parameters based on Bennett and Rabbetts’ schematic eye[40] into these formulas, the second principal point
position ranges from 2.67 to 1.46. As a result, the q value in
Littmann’s formula modified by Bennett et al[29],
which expresses the magnification factor of the individual eye, varies by
-0.0048 (-1.6%) to 0.0111 (+3.8%) compared to average axial length of 24.39 mm
and the second principal point of 1.82 mm. Therefore, the apparent ONH size on
a fundus photograph might be slightly decreased with induced increases in
corneal power, decreasing cpRNFL and ONH measurements. However, the second
principal point position was calculated with approximate value based on the
schematic eyes, not actually measured in each participant. Further study is
needed using the actual value in each participant.
is the distance from the anterior surface
of the cornea to the second principal plane of the eye (second principal point).
By substituting the variation value of corneal power in the current study and
other parameters based on Bennett and Rabbetts’ schematic eye[40] into these formulas, the second principal point
position ranges from 2.67 to 1.46. As a result, the q value in
Littmann’s formula modified by Bennett et al[29],
which expresses the magnification factor of the individual eye, varies by
-0.0048 (-1.6%) to 0.0111 (+3.8%) compared to average axial length of 24.39 mm
and the second principal point of 1.82 mm. Therefore, the apparent ONH size on
a fundus photograph might be slightly decreased with induced increases in
corneal power, decreasing cpRNFL and ONH measurements. However, the second
principal point position was calculated with approximate value based on the
schematic eyes, not actually measured in each participant. Further study is
needed using the actual value in each participant.
A slight difference in cpRNFL thickness was noted between the previous (−0.4
to −0.5 μm/diopter)[30-31] and
the current (−0.2 to −0.3 μm/diopter) studies. This difference can be
attributed to the control of accommodative effects by cycloplegic eye drops.
Although cycloplegic eye drops were used to control pupil size and
accommodation in previous studies[30-31],
the cpRNFL and ONH were imaged without cycloplegia in the current study. The
anterior pole of the lens moves anteriorly by 0.05 mm/diopter of accommodation,
while the posterior pole moves slightly back by 0.01 mm/diopter; thus, the
center of the lens moves forward by 0.02 mm/diopter. This means that 0.24 mm of
the 1.2 mm range of the second principal point change may be a direct result of
the lens anterior shift as a consequence of the accommodation in this study.
Further, the position of the second principal point would have varied slightly
due to accommodation that occurred when the corneal power was decreased by
using the contact lenses with a high negative power.
Previous studies showed that measured cpRNFL thickness without
correction for ocular magnification decreases in the range of −1.8 to −4.8 μm as the 1-mm axial length increases[13,15-17,19,21-22,25,27]. These
slope values can be converted to −0.6 to −1.6 per diopter using a ratio of 1 mm
axial length to 3-diopter refractive error based on a three-surface schematic
eye[40]. In addition, the measured disc area
without correction for ocular magnification becomes smaller by −0.72 mm2
as myopia increases by 1 diopter[26]. Although
the results cannot be directly compared because the previous data are based on
interindividual comparisons[13,15-17,19,21-22,25-27], they suggest that the
influence of corneal power on cpRNFL and ONH measurements is less than that of
axial length.
There was no difference in cpRNFL thickness in the nasal region induced
by an increase in corneal power. The magnitude of curvature in this region is generally
larger than that of the temporal, superior, or inferior region, especially
considering the longer axial length of a myopic eye. The cpRNFL thickness was
measured by the same scan circle size. When the fundus image is magnified by
the induced increase in corneal power, the scan area at the nasal region is
smaller than that of the temporal, superior, or inferior region. No difference
in cpRNFL thickness at the nasal region could be attributed to the magnitude of
curvature of the fundus since the scan circle is centered on the optic disc.
Research on refractive surgeries for myopia such as laser-assisted in
situ keratomileusis[41-45],
small incision lenticule extraction[46-51], and phakic intraocular lens implantation[52-54] has been performed worldwide.
Although these procedures could change the position of the second principal
point, a previous report indicated that refractive surgery does not affect the
measured cpRNFL thickness[55-57].
A reason for this finding is that ocular magnification does not change
considerably because the cornea is minimally resected. However, careful
attention is required for ocular magnification when the corneal resection
volume is large.
In summary, induced changes in corneal power lead to decreased cpRNFL
and ONH measurements in SD-OCT. For more precise OCT imaging, the ocular
magnification should be corrected by considering the individual axial length
and second principal point position. However, the conventional magnification
correction based on Littmann’s formula modified by Bennett et al[29] is adequate for daily clinical imaging because the
apparent changes in cpRNFL thickness and ONH topography due to corneal power
changes are small when compared with those due to axial length.
Acknowledgements
Foundation: Supported by a Research
Fund at Kitasato University.
Conflicts of Interest: Hirasawa K, None; Shoji N, None.
References
1 Leung CK, Cheung CY, Weinreb RN, Qiu Q, Liu S, Li H, Xu G,
Fan N, Huang L, Pang CP, Lam DS. Retinal nerve fiber layer imaging with
spectral-domain optical coherence tomography: a variability and diagnostic
performance study. Ophthalmology 2009;116(7):1257-1263,
1263.e1-e2.
2 Mwanza JC,
Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, Feuer WJ. Reproducibility of
peripapillary retinal nerve fiber layer thickness and optic nerve head
parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci
2010;51(11):5724-5730. [CrossRef]
[PMC free
article] [PubMed]
3 Garcia-Martin
E, Pinilla I, Idoipe M, Fuertes I, Pueyo V. Intra and interoperator
reproducibility of retinal nerve fibre and macular thickness measurements using
Cirrus Fourier-domain OCT. Acta
Ophthalmol 2011; 89(1):e23-e29. [CrossRef] [PubMed]
4 Kim JS,
Ishikawa H, Sung KR, Xu J, Wollstein G, Bilonick RA, Gabriele ML, Kagemann L,
Duker JS, Fujimoto JG, Schuman JS. Retinal nerve fibre layer thickness
measurement reproducibility improved with spectral domain optical coherence
tomography. Br J Ophthalmol 2009;93(8):1057-1063. [CrossRef] [PMC free article]
[PubMed]
5 Carpineto P,
Nubile M, Agnifili L, Toto L, Aharrh-Gnama A, Mastropasqua R, Di Antonio L,
Fasanella V, Mastropasqua A. Reproducibility and repeatability of CirrusTM
HD-OCT peripapillary retinal nerve fibre layer thickness measurements in young
normal subjects. Ophthalmologica
2012;227(3):139-145. [CrossRef]
[PubMed]
6 Tan BB,
Natividad M, Chua KC, Yip LW. Comparison of retinal nerve fiber layer
measurement between 2 spectral domain OCT instruments. J Glaucoma 2012;21(4):266-273. [CrossRef] [PubMed]
7 Wu H, de
Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements
using spectral domain optical coherence tomography. J Glaucoma 2011;20(8):470-476. [PMC free article]
[PubMed]
8
Gonzalez-Garcia AO, Vizzeri G, Bowd C, Medeiros FA, Zangwill LM, Weinreb RN.
Reproducibility of RTVue retinal nerve fiber layer thickness and optic disc
measurements and agreement with Stratus optical coherence tomography
measurements. Am J Ophthalmol 2009;147(6):1067-1074, 1074.e1061.
9 Garas A,
Vargha P, Hollo G. Reproducibility of retinal nerve fiber layer and macular
thickness measurement with the RTVue-100 optical coherence tomograph. Ophthalmology 2010;117(4):738-746. [CrossRef] [PubMed]
10 Nakatani Y,
Higashide T, Ohkubo S, Takeda H, Sugiyama K. Evaluation of macular thickness
and peripapillary retinal nerve fiber layer thickness for detection of early
glaucoma using spectral domain optical coherence tomography. J Glaucoma 2011;20(4):252-259. [CrossRef] [PubMed]
11 Lee SH, Kim
SH, Kim TW, Park KH, Kim DM. Reproducibility of retinal nerve fiber thickness
measurements using the test-retest function of spectral OCT/SLO in normal and
glaucomatous eyes. J Glaucoma 2010;
19(9):637-642. [CrossRef]
[PubMed]
12 Mansoori T,
Viswanath K, Balakrishna N. Reproducibility of peripapillary retinal nerve
fibre layer thickness measurements with spectral domain optical coherence
tomography in normal and glaucomatous eyes. Br
J Ophthalmol 2011;95(5):685-688. [CrossRef] [PubMed]
13 Hirasawa K,
Shoji N, Yoshii Y, Haraguchi S. Determination of axial length requiring
adjustment of measured circumpapillary retinal nerve fiber layer thickness for
ocular magnification. PLoS One
2014;9(9):e107553. [CrossRef]
[PMC free
article] [PubMed]
14 Miki A,
Medeiros FA, Weinreb RN, Jain S, He F, Sharpsten L, Khachatryan N, Hammel N, Liebman
JM, Girkin CA, Sample PA, Zengwill LM. Rates of retinal nerve fiber layer
thinning in glaucoma suspect eyes. Ophthalmology
2014;121(7):1350-1358. [CrossRef]
[PMC free
article] [PubMed]
15 Leung CK,
Mohamed S, Leung KS, Cheung CY, Chan SL, Cheng DK, Lee AK, Leung GY, Rao SK,
Lam DS. Retinal nerve fiber layer measurements in myopia: an optical coherence
tomography study. Invest Ophthalmol Vis
Sci 2006;47(12):5171-5176. [CrossRef]
[PubMed]
16 Budenz DL,
Anderson DR, Varma R, Schuman J, Cantor L, Savell J, Greenfield DS, Patella VM,
Quigley HA, Tielsch J. Determinants of normal retinal nerve fiber layer
thickness measured by Stratus OCT. Ophthalmology
2007;114(6):1046-1052. [CrossRef]
[PMC free
article] [PubMed]
17 Hougaard
JL, Ostenfeld C, Heijl A, Bengtsson B. Modelling the normal retinal nerve fibre
layer thickness as measured by Stratus optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2006;
244(12):1607-1614. [CrossRef]
[PubMed]
18 Kim MJ, Lee
EJ, Kim TW. Peripapillary retinal nerve fibre layer thickness profile in subjects
with myopia measured using the Stratus optical coherence tomography. Br J Ophthalmol 2010;94(1):115-120. [CrossRef] [PubMed]
19
Bendschneider D, Tornow RP, Horn FK, Laemmer R, Roessler CW, Juenemann AG,
Kruse FE, Mardin CY. Retinal nerve fiber layer thickness in normals measured by
spectral domain OCT. J Glaucoma 2010;19(7):475-482.
[CrossRef] [PubMed]
20 Cheung CY,
Chen D, Wong TY, Tham YC, Wu R, Zheng Y, Cheng CY, Saw SM, Baskaran M, Leung
CK, Aung T. Determinants of quantitative optic nerve measurements using
spectral domain optical coherence tomography in a population-based sample of
non-glaucomatous subjects. Invest Ophthalmol
Vis Sci 2011;52(13):9629-9635. [CrossRef] [PubMed]
21 Yoo YC, Lee
CM, Park JH. Changes in peripapillary retinal nerve fiber layer distribution by
axial length. Optom Vis Sci
2012;89(1):4-11. [CrossRef]
[PubMed]
22 Huang D,
Chopra V, Lu AT, Tan O, Francis B, Varma R. Advanced Imaging for Glaucoma Study-AIGS
Group. Does optic nerve head size variation affect circumpapillary retinal
nerve fiber layer thickness measurement by optical coherence tomography? Invest Ophthalmol Vis Sci
2012;53(8):4990-4997. [CrossRef]
[PMC free
article] [PubMed]
23 Aykut V,
Oner V, Tas M, Iscan Y, Agachan A. Influence of axial length on peripapillary
retinal nerve fiber layer thickness in children: a study by RTVue
spectral-domain optical coherence tomography. Curr Eye Res 2013;38(12):1241-1247. [CrossRef] [PubMed]
24 Oner V,
Aykut V, Tas M, Alakus MF, Iscan Y. Effect of refractive status on
peripapillary retinal nerve fibre layer thickness: a study by RTVue spectral
domain optical coherence tomography. Br J
Ophthalmol 2013;97(1):75-79. [CrossRef] [PubMed]
25 Kang SH,
Hong SW, Im SK, Lee SH, Ahn MD. Effect of myopia on the thickness of the retinal
nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci 2010;51(8):
4075-4083. [CrossRef] [PubMed]
26 Savini G,
Barboni P, Parisi V, Carbonelli M. The influence of axial length on retinal
nerve fibre layer thickness and optic-disc size measurements by spectral-domain
OCT. Br J Ophthalmol 2012;96(1):57-61.
[CrossRef] [PubMed]
27 Hirasawa K,
Shoji N, Yoshii Y, Haraguchi S. Comparison of Kang's and Littmann's methods of
correction for ocular magnification in circumpapillary retinal nerve fiber
layer measurement. Invest Ophthalmol Vis
Sci 2014;55(12):8353-8358. [CrossRef]
[PubMed]
28 Littmann H.
Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd
1982;180(4):286-289. [CrossRef]
[PubMed]
29 Bennett AG,
Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the
size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol 1994;232(6):361-367. [CrossRef]
30 Lee J, Kim
NR, Kim H, Han J, Lee ES, Seong GJ, Kim CY. Negative refraction power causes
underestimation of peripapillary retinal nerve fibre layer thickness in
spectral-domain optical coherence tomography. Br J Ophthalmol 2011;95(9):1284-1289. [CrossRef] [PubMed]
31 Patel NB,
Garcia B, Harwerth RS. Influence of anterior segment power on the scan path and
RNFL thickness using SD-OCT. Invest
Ophthalmol Vis Sci 2012;53(9):5788-5798. [CrossRef] [PMC free article]
[PubMed]
32 Salchow DJ,
Hwang AM, Li FY, Dziura J. Effect of contact lens power on optical coherence
tomography of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci 2011;52(3):1650-1654. [CrossRef] [PubMed]
33 Faul F,
Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1:
tests for correlation and regression analyses. Behav Res Methods 2009;41(4):1149-1160. [CrossRef] [PubMed]
34 Faul F,
Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power
analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175-191. [CrossRef]
35 Cohen J.
Statistical power analysis for the behavioral sciences. Second Edition.
Hillsdale, New Jersey: Lawrence Erlbaum Associates, 1988.
36 Bland JM,
Altman DG. Measurement error proportional to the mean. BMJ 1996;313(7049):106. [CrossRef] [PMC free article]
[PubMed]
37 Bland JM,
Altman DG. Measurement error. BMJ
1996;313(7059):744. [CrossRef]
[PMC free
article] [PubMed]
38 Cheung CY,
Leung CK, Lin D, Pang CP, Lam DS. Relationship between retinal nerve fiber
layer measurement and signal strength in optical coherence tomography. Ophthalmology 2008;115(8):1347-1351,
1351.e1-e2.
39 Ha MM, Kim
JM, Kim HJ, Park KH, Kim M, Choi CY. Low limit for effective signal strength in
the Stratus OCT in imperative low signal strength cases. Korean J Ophthalmol 2012;26(3):182-188. [CrossRef] [PMC free article]
[PubMed]
40 Rabbett RB.
Bennett and Rabbetts' Clinical Visual Optics. 4th Edition. Oxford:
Butterworth-Heinemann, 2007.
41 Aizawa D,
Shimizu K, Komatsu M, Ito M, Suzuki M, Ohno K, Uozato H. Clinical outcomes of
wavefront-guided laser in situ keratomileusis: 6-month follow-up. J Cataract Refract Surg
2003;29(8):1507-1513. [CrossRef]
42 Igarashi A,
Kamiya K, Shimizu K, Komatsu M. Visual performance after implantable collamer
lens implantation and wavefront-guided laser in situ keratomileusis for high
myopia. Am J Ophthalmol 2009;148(1):
164-170.e1. [CrossRef]
[PubMed]
43 Igarashi A,
Kamiya K, Shimizu K, Komatsu M. Time course of refractive and corneal
astigmatism after laser in situ keratomileusis for moderate to high
astigmatism. J Cataract Refract Surg 2012;38(8):1408-1413. [CrossRef] [PubMed]
44 Kamiya K,
Shimizu K, Igarashi A, Komatsu M. Comparison of Collamer toric implantable
[corrected] contact lens implantation and wavefront-guided laser in situ
keratomileusis for high myopic astigmatism. J
Cataract Refract Surg 2008;34(10):1687-1693. [CrossRef] [PubMed]
45 Kobashi H,
Kamiya K, Igarashi A, Matsumura K, Komatsu M, Shimizu K. Long-term quality of
life after posterior chamber phakic intraocular lens implantation and after
wavefront-guided laser in situ keratomileusis for myopia. J Cataract Refract Surg 2014;40(12):2019-2024. [CrossRef] [PubMed]
46 Ishii R,
Shimizu K, Igarashi A, Kobashi H, Kamiya K. Influence of femtosecond lenticule
extraction and small incision lenticule extraction on corneal nerve density and
ocular surface: a 1-year prospective, confocal, microscopic study. J Refract Surg 2015;31(1):10-15. [CrossRef] [PubMed]
47 Kamiya K,
Shimizu K, Igarashi A, Kobashi H. Visual and refractive outcomes of femtosecond
lenticule extraction and small-incision lenticule extraction for myopia. Am J Ophthalmol 2014;157(1):128-134.e2.
[CrossRef] [PubMed]
48 Kamiya K,
Shimizu K, Igarashi A, Kobashi H. Effect of femtosecond laser setting on visual
performance after small-incision lenticule extraction for myopia. Br J Ophthalmol 2015;99(10):1381-1387. [CrossRef] [PubMed]
49 Kamiya K,
Shimizu K, Igarashi A, Kobashi H, Sato N, Ishii R. Intraindividual comparison
of changes in corneal biomechanical parameters after femtosecond lenticule
extraction and small-incision lenticule extraction. J Cataract Refract Surg 2014;40(6):963-970. [CrossRef] [PubMed]
50 Kobashi H,
Kamiya K, Ali MA, Igarashi A, Elewa ME, Shimizu K. Comparison of astigmatic
correction after femtosecond lenticule extraction and small-incision lenticule
extraction for myopic astigmatism. PLoS
One 2015;10(4):e0123408. [CrossRef] [PMC free article]
[PubMed]
51 Sekundo W,
Kunert KS, Blum M. Small incision corneal refractive surgery using the small
incision lenticule extraction (SMILE) procedure for the correction of myopia
and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol 2011;95(3):335-339. [CrossRef] [PubMed]
52 Igarashi A,
Shimizu K, Kamiya K. Eight-year follow-up of posterior chamber phakic intraocular
lens implantation for moderate to high myopia. Am J Ophthalmol 2014;157(3):532-539.e1. [CrossRef] [PubMed]
53 Kamiya K,
Shimizu K, Igarashi A, Kobashi H. Factors influencing long-term regression
after posterior chamber phakic intraocular lens implantation for moderate to
high myopia. Am J Ophthalmol 2014; 158(1):179-184.e1.
[CrossRef] [PubMed]
54 Kamiya K,
Shimizu K, Kobashi H, Igarashi A, Komatsu M, Nakamura A, Kojima T, Nakamura T.
Three-year follow-up of posterior chamber toric phakic intraocular lens
implantation for the correction of high myopic astigmatism in eyes with
keratoconus. Br J Ophthalmol
2015;99(2):177-183. [CrossRef]
[PubMed]
55
Gurses-Ozden R, Liebmann JM, Schuffner D, Buxton DF, Soloway BD, Ritch R.
Retinal nerve fiber layer thickness remains unchanged following laser-assisted
in situ keratomileusis. Am J Ophthalmol
2001;132(4):512-516. [CrossRef]
56 Sharma N,
Sony P, Gupta A, Vajpayee RB. Effect of laser in situ keratomileusis and
laser-assisted subepithelial keratectomy on retinal nerve fiber layer
thickness. J Cataract Refract Surg 2006;32(3):446-450. [CrossRef] [PubMed]
57 Zangwill
LM, Abunto T, Bowd C, Angeles R, Schanzlin DJ, Weinreb RN. Scanning laser
polarimetry retinal nerve fiber layer thickness measurements after LASIK. Ophthalmology 2005;112(2):200-207. [CrossRef] [PubMed]
--------------------------------------------------------------------------------------------------------------------------------
All rights reserved by Press of International Journal of Ophthalmology (IJO
PRESS)