Age related changes of the central lamina cribrosa thickness, depth and prelaminar tissue in healthy Chinese subjects
Hui Xiao, Xiao-Yu Xu, Yi-Min Zhong, Xing Liu
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sun University, Guangzhou 510060, Guangdong Province, China
Abstract
● AlM:To investigate the variation in the central lamina cribrosa thickness (cLCT), and the central anterior lamina cribrosa surface depth (cALCSD), as well as the central prelaminar tissue thickness (cPLTT) related to age in healthy Chinese subjects.
● METHODS:A total of 96 eyes from 96 Chinese healthy subjects were recruited.According to age, the 96 cases were divided into three groups: the young group (YG,18-39y), middle-age group (MG, 40-59y) and older-age group (OG, 60y and above).Lamina cribrosa images were obtained from all participants using radial linear protocol by enhanced depth imaging spectral-domain optical coherence tomography.The cLCT, cALCSD and cPLTT were calculated from the average value of the lamina cribrosa thickness, anterior lamina cribrosa surface depth and prelaminar tissue thickness in the optic nerve head(ONH) centre point and paracentral points (150 μm from the centre point in the horizontal and vertical directions).
● RESULTS:For the total subjects, the mean cLCT,cALCSD and cPLTT were 235.18±41.27, 358.02±93.80 and 182.02±92.11 μm, respectively.No statistically significant differences in cLCT, cALCSD or cPLTT were found between gender and different eyes (P=0.27-0.92).The cLCT of the OG was the thickest among the three groups, while the cPLTT of the YG was the thickest among the three groups(P<0.05).Age was positively correlated with cLCT (r=0.42,P<0.001), and negatively correlated with cPLTT (r=-0.24,P=0.02).No signifcant correlation was found between the age and cALCSD (r=-0.06,P=0.55).And no correlation has been found between axial length and cLCT, cALCSD and cPLTT (P=0.11-0.81).
● CONCLUSlON:The impact of age on the cLCT and the cPLLTT should be taken into account when analysing glaucoma and other diseases related to lamina cribrosa.
● KEYWORDS:lamina cribrosa; thickness; normal subjects;optical coherence tomography; age
INTRODUCTION
Lamina cribrosa (LC) is located at the posterior pole of the eye.It inserts into the sclera canal wall but is not continuous with the sclera.Such discontinuities between the LC and sclera make the LC a weak point of stress and strain[1],and makes it susceptible to damage from increased intraocular pressure (IOP) and translaminar pressure gradient[2-3].Quigleyet al[4]used the scanning electron microscopy and found that in glaucomatous eyes the LC sheets were compressed and the entire LC was backward bowing and considered the compression of LC might be a primary pathogenetic event in glaucomatous damage.Levyet al’s[5]study observed laminar posterior displacement after IOP elevation in primate eye.In experimental glaucoma monkey, the posterior deformation and thickening of the lamina were documented in early glaucoma eyes[6].Because the LC is buried deeply beneath the optic nerve head (ONH) and difficult to be observed directly, previous studies of the LC have been limited to animal experiments or histological observations of cadaver eyes until the application of the scanning mode of enhanced depth imaging (EDI) by spectral-domain optical coherence tomography (SD-OCT)[5-7].Thein vivostudies of LC revealed that the thickness of central LC was thinner in glaucomatous eyes than that of normal controls[8]and the depth of LC was signifcantly deeper than that of normal controls[9].Epidemiological surveys have indicated that the incidence of glaucoma increases with age[10],thus investigate the influence of age on the LC may help to understand the pathogenesis of glaucoma.The presentin vivostudy collected the LC images from healthy Chinese subjects of different ages, and investigated the age related changes of the central lamina cribrosa thickness (cLCT), the central anterior lamina cribrosa surface depth (cALCSD) and the central prelaminar tissue thickness (cPLTT).
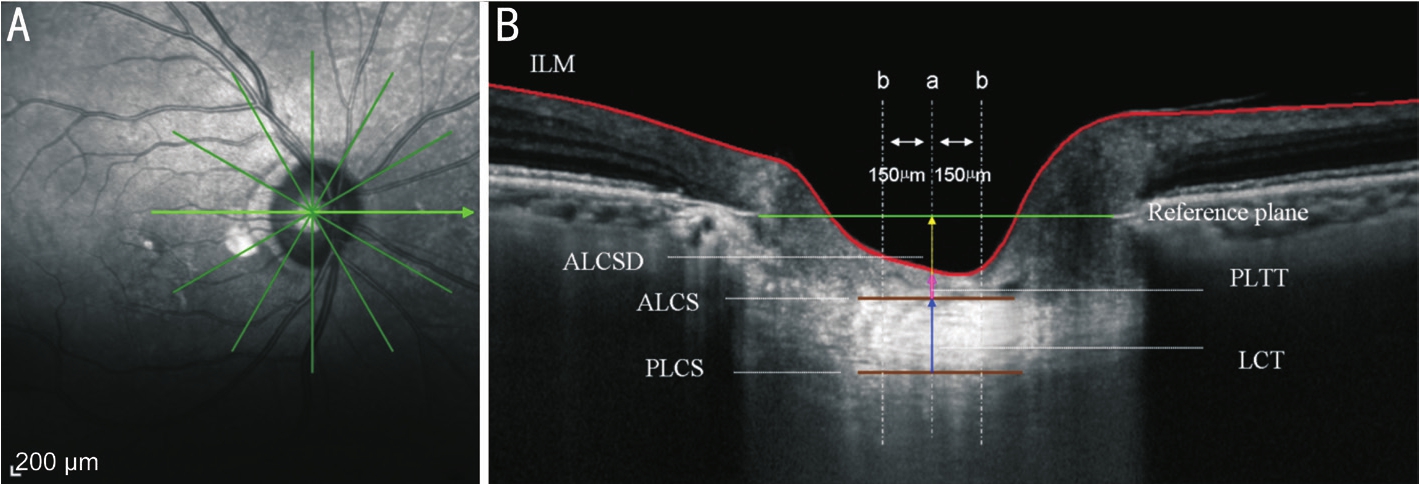
Figure 1 Illustration of the definition of cLCT, cPLTT, and cALCSD in the horizontal direction A: Infrared imaging of the optic disc with radial scanning protocol; B: OCT image of LC.The green line presents the connection of the terminal of Bruch’s membrane; the brown lines represent ALCS and PLCS; the blue narrow present LCT; the red line presents the inner limiting membrane (ILM); the pink narrow presents PLTT; the yellow arrows presents ALCSD; the three gray dotted lines present different LC measure points.aONH center measure point;bParacentral measure points.
SUBJECTS AND METHODS
This study was conducted in the Zhongshan Ophthalmic Center, Sun Yat-sen University.The research protocols were approved by the Institutional Review Board at Zhongshan Ophthalmic Center and carried out in accordance with the tenets of the Declaration of Helsinki.Written informed consent was obtained from all enrolled participants.A total of 96 healthy volunteers with no history of eye disorders were recruited in this study, including 51 males and 45 females.
The inclusion criteria were: 1) best-corrected visual acuity(BCVA) ≥16/20; 2) normal result on standard automated perimetry; 3) IOP<21 mm Hg with no history of increased IOP; 4) cup/disc (C/D ratio) <0.6, with no retinal nerve fber layer (RNFL) defects.Exclusion criteria were: 1) spherical equivalent >±6 diopters (D), and astigmatism >±3 D; 2)patients with diseases affecting the LC or the optic nerve such as glaucoma or ischaemic optic neuropathyetc; 3) poor image quality affecting the recognition of the boundary of the LC.The 96 cases were divided into three groups according to age:the young group (YG), middle-age group (MG) and older-age group (OG).The YG was defned as subjects aged 18-39y; the MG was defned as subjects aged 40-59y; the OG was defned as subjects aged 60y and above.
All subjects received complete ophthalmic examinations,which included the BCVA easurement, Goldmann applanation tonometry, slit-lamp biomicroscopy, gonioscopy, visual field examination, axial length (AL) measurement and dilated stereoscopic examination of the optic disc.AL was determined using A-scan ultrasound (Cinescan A/Bscan; Quantel Medical,Clemon, France).AL was defned as the distance between the cornea and the retina.
For each eligible patient, one eye was randomly chosen to be scanned using an EDI system of Heidelberg Spectralis optical coherence tomography (OCT) (wavelength: 870 nm;scan pattern: EDI; Spectralis Viewing Module version 6.0.9.0;Heidelberg Engineering, Heidelberg, Germany).The radial scanning protocol comprising 6 angularly equidistant linear scans centering at the centre of ONH was conducted with a scan angle of 20° (Figure 1A).The EDI image was averaged for 100 scans using the automatic averaging and eye tracking system to minimize the noise and created the highest imaging quality.Clear images were selected and saved.The LC appeared as a highly reflective plate-like structure in B-scan images.The anterior and posterior surfaces of the LC were considered to be where the highly reflective region started and ended within the ONH[11].The centre of the ONH was identified as the midpoint between the Bruch’s membrane opening.The lamina cribrosa thickness (LCT) was defined as the distance between the anterior lamina cribrosa surface(ALCS) and posterior lamina cribrosa surface (PLCS).The cLCT was calculated from the average value of the LCT in the ONH centre point and paracentral points (150 μm from the centre point in the horizontal and vertical directions).The anterior lamina cribrosa surface depth (ALCSD) was defned as the distance between the ALCS and the reference plane(the connection of the terminal of Bruch’s membrane were defned as the reference plane).The cALCSD was calculated from the average value of ALCSD in the ONH centre point and paracentral points (150 μm from the centre point in the horizontal and vertical directions).The prelaminar tissue thickness (PLTT) was defined as the distance between the ALCS and the bottom of the optic cup.The cPLTT was calculated from the average value of PLTT in the ONH centre point and paracentral points (150 μm from the centre point in the horizontal and vertical directions) (Figure 1B).The cLCT,cALCSD and cPLTT in the ONH centre and paracentral points were measured by the same experienced doctor (Xiao H).
To evaluate the inter-observer repeatability of our measuring method, 20 eyes from the 96 subjects were selected to be measured independently by two different observers (A and B).And observer A who was blinded to the previous measurements, performed an additional measurement on another day for determination of intra-observer repeatability(A1 and A2).
Statistical AnalysisThe data were analysed by a commercial analytical software program (SPSS 16.0; SPSS, Inc., Chicago,IL, USA).The inter-observer and intra-observer measurement standard deviation (SD), coefficient of variation (CV), and intra-class correlation coeffcient (ICC) were used to evaluate the intra-observer and the inter-observer repeatability of our measurement method.Kolmogorov-Smirov test and Levene test were conducted to test the data normality and homogeneity of variance, respectively.Independentt-tests were used to compare the cLCT, cALCSD, and cPLTT between different gender and eyes.Analysis of covariance (ANCOVA) was applied to compare the average cLCT, cALCSD, and cPLTT measurements among the three different age groups in order to exclude the mixed effect of AL.A Pearson correlation analysis was calculated for variation in the three LC parameters (LCT,PLTT and ALCSD) relative to age and AL.P<0.05 was considered to be statistically signifcant.
RESULTS
The repeatability of inter-observer and intra-observer measurement of cLCT, cALCSD and cPLTT was obtained from 20 eyes.The inter-observer and intra-observer measurement SD, CV, and ICC are listed as Table 1.
There were 51 males and 45 females included in the study.The mean overall age was 50.34±18.20 (range 18-80)y.No statistically signifcant differences were noted in age between males and females (P=0.64).The mean refractive error was-1.25±1.68 D (-4.50-1.0 D).The mean AL was 23.98±1.24 mm(range 22.52-24.96 mm).The mean overall cLCT, cALCSD and cPLTT were 235.18±41.27 (95%CI 226.50-243.51) μm,358.02±93.80 (95%CI 339.01-377.03) μm, and 182.02±92.11(95%CI 163.33-200.68) μm, respectively.No statistically significant differences in cLCT, cALCSD and cPLTT were found between genders (P=0.27-0.92).There were 47 right eyes and 49 left eyes included in the study.No statistically significant differences in cLCT, cALCSD and cPLTT were found between different eyes (P=0.36-0.59; Table 2).
The YG included 29 eyes of 29 cases, with an average age of 26.72±6.26y (range 18-39y); The MG included 25 eyes of 25 cases, with an average age of 49.44±5.64y (rang 41-58y);The OG included 42 eyes of 42 cases, with an average age of 67.19±6.04y (rang 60-80y).The mean cLCTs of the three groups were 213.38±25.50 μm, 228.16±28.89 μm, and 254.42±47.62 μm, respectively, with statistically significant differences among the three groups (P<0.001).The mean cALCSDs of the three groups were 364.78±86.22 μm,386.24±127.05 μm, and 336.32±79.71 μm, respectively.No statistically significant differences among the three groups were found in cALCSD (P=0.11).The mean cPLTTs of the three groups were 224.70±114.84 μm, 174.16±121.27 μm and 159.80±74.05 μm respectively, with statistically significant differences among the three group (P<0.05; Figure 2).Post Hoc analysis using least signifcant difference test showed the cLCT of the OG was thicker than that of YG (P<0.001) and MG (P=0.007), while the cPLTT of the YG was thicker than that of the MG (P=0.03) and OG (P<0.001).
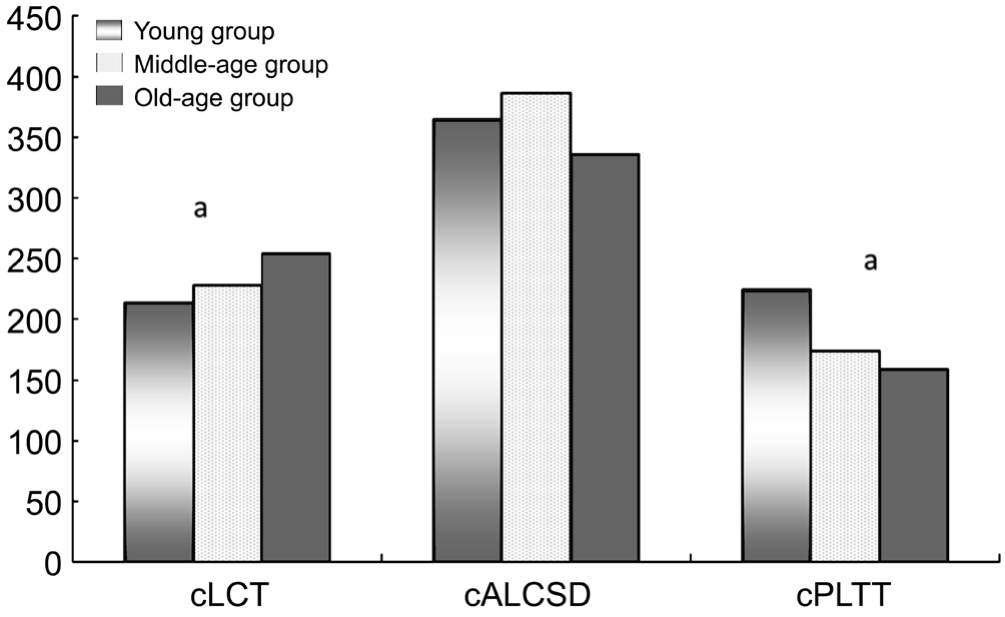
Figure 2 cLCT, cALCSD and cPLTT in three different age groups
aANCOVA showed statistically significant differences among the three age groups (P<0.05).
Table 1 Repeatability of inter-observer and intra-observer measurements
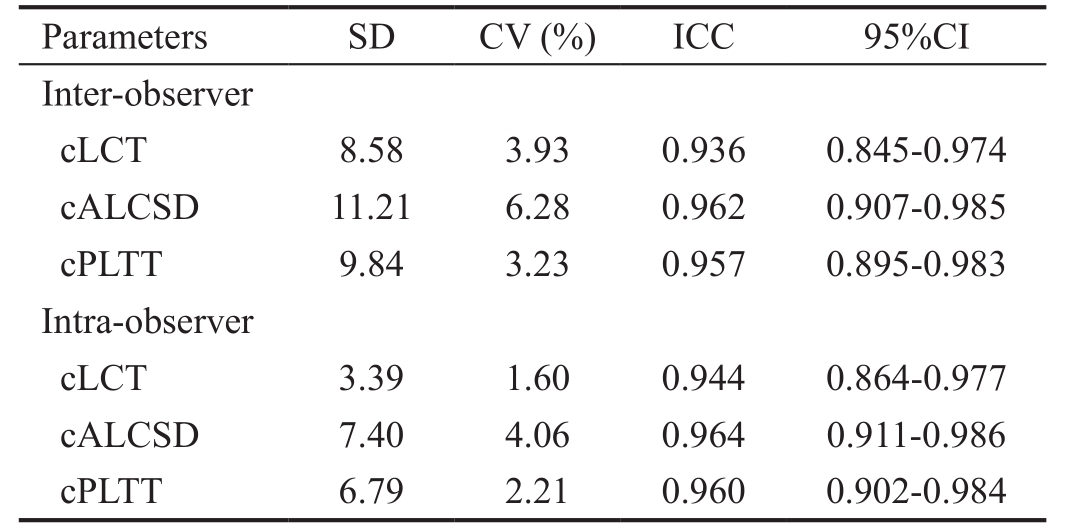
cLCT: Central lamina cribrosa thickness; cALCSD: Central anterior lamina cribrosa surface depth; cPLTT: Central prelaminar tissue thickness; CV: Coefficient of variation; ICC: Intra-class correlation coeffcient.
Parameters SD CV (%) ICC 95%CI Inter-observer cLCT 8.58 3.93 0.936 0.845-0.974 cALCSD 11.21 6.28 0.962 0.907-0.985 cPLTT 9.84 3.23 0.957 0.895-0.983 Intra-observer cLCT 3.39 1.60 0.944 0.864-0.977 cALCSD 7.40 4.06 0.964 0.911-0.986 cPLTT 6.79 2.21 0.960 0.902-0.984
Correlation analysis revealed that age was positively correlated with cLCT (r=0.42,P<0.001, with 95%CI 0.22-0.62) and negatively correlated with cPLTT (r=-0.24,P=0.02 with 95%CI -0.35 to -0.13).Age and cALCSD were not signifcantly correlated (r=-0.06,P=0.55).No correlation was found between AL and age (P=0.17), and no correlation was found between AL and cLCT, cPLTT as well as cALCSD(P=0.62, 0.81, 0.11 respectively).
DISCUSSION
Previous study[11]defned the structure of the LC on an OCT image as the highly reflective region containing numerous pores in the deep area of ONH.Due to the shadow of sclera and nerve tissue, pigment of the retina or choroid, or blood vessels, the details of the peripheral LC were obscured anddiffcult to be identifed the boundary.Therefore, the present study only focused on the LC in the centre of the ONH.The ICCs of inter-observer and intra-observe measurement of the three central laminar parameters both reach 0.9 indicating that the central laminar parameters measurement method was reliable.
Table 2 LC parameters between different gender and eyes

cLCT: Central lamina cribrosa thickness; cALCSD: Central anterior lamina cribrosa surface depth; cPLTT: Central prelaminar tissue thickness.
Parameters (μm) GenderPDifferent eyePF (n=51) M (n=45) Right (n=47) Left (n=49)cLCT 235.43±44.60 234.53±39.30 0.92 237.86±44.95 232.04±38.91 0.50 cALCSD 367.92±93.58 346.80±93.82 0.27 366.63±92.21 349.04±95.59 0.36 cPLTT 191.71±101.12 171.04±80.41 0.28 187.04±96.61 176.79±87.90 0.59
Renet al[12]reported that the LCT of enucleated eyes from normal Chinese people was 207±60 μm, which is thinner than that of present study (235.18±41.27 μm).This difference in cLCT may be attributed to the different study methods.The LCT in enucleated globes was examined after being fxated for histopathological analysis, and it is known that the methods used for histologic preparation may induce tissue swelling or shrinkage[12-13].Therefore, their data may be smaller than thein vivovalue due to the dehydration.Kotechaet al[7]has reported that the LCT of normal subjects ranged from 345.4 to 555.9 μm,which was thicker than the result of the present study.This difference may be due to the measurements were conducted in different ethnic groups.Leeet al[14]reported that the cLCT of normal Korean people obtained by EDI SD-OCTin vivowas 273.19±34.74 μm which was thicker than our result.However,their measurement was the average of the horizontal scanning on the centre of the LC and the horizontal scanning at the above and below adjacent areas.The cLCT measurement in the present study was calculated from the average value of the LCT in the ONH centre point and paracentral points in the horizontal and vertical direction.These different averaging methods may lead to the slight difference in these results.
Kotechaet al[7]reported that the LCT was thicker with age which based on a pathological study of cadaver eyes from normal subjects.Immunohistochemical and biochemical studies have confirmed the accumulation of collagen in the LC beginning at birth and continuing throughout the whole lifespan[15-16].The presentin vivostudy revealed that LCT was thicker in the older subjects, especially in the old people above 60y which seems consistent with the pathology and immunohistochemical studies.Anin vivostudy conducted by Leeet al[14]also confirmed that the LCT was increased with age.The thickening of LC is likely to contribute to the stiffened connective tissues and reduced compliance of LC with age[17], leading to increased susceptibility to IOP-induced glaucomatous injury in the elderly.However, Parket al[8]compared patients with normal-tension glaucoma and hightension glaucoma to normal subjects and found that the LC in the glaucoma patients was significantly thinner than in the normal subjects.Chunget al[18]even found thinner baseline LCT was independently associated with glaucoma progression in their study.But in the experimental glaucoma in monkeys, thickening of LC has been documented in the monkey with early glaucomatous eyes[6].These contradictory findings indicate the complex relationship between LC thickening, stiffening, and IOP-related axonal insult.It is less well understood how the molecular and biochemical characterization of early glaucoma leads to changes within the LC, but age-related laminar changes may play an important role in the progression of laminar morphology from a normal state to that of a cupped, excavated glaucomatous state and further study are needed.
Sigalet al[19]reported the LC insertion into the pia mater did not increase with age in the donor eyes.However, Rhodeset al[20]found that the depth of LC changes with age differently across racial groups in normal subjects.The laminar depth in European descent subjects showed a significantly decreasing with age, but this phenomenon was not found in people of African descent.We observed a group of healthy Chinese subjects and also found no correlation between cALCSD and age.A study in high-risk ocular hypertension and glaucoma patients by Renet al[21]demonstrated that the cALCSD in older eyes was shallower than in younger eyes for the same visual field status and average RNFL thickness.It seems the cALCSD becomes shallow with age in glaucoma and suspect glaucoma patient.The contradictory results may be due to the compliance of LC at different age.It is should be note that Renet al’s[21]study was obtained in patient with high ocular pressure.Increased IOP will lead to LC deformation and bowing backward[22].The ability to deformation depended on the compliance of LC[23].However, the compliance of LC is reduced with age[15], thus the bowing backward will be much less in glaucoma patients with older age than younger eyes for the same visual field status and RNFL thickness.When it comes to normal healthy people, without the pathological displacement, the original depth of LC may not be affected by age.Moreover, it also should be noted that most of the previousin vivostudies of the lamina used the Bruch’s membrane as a reference plane when measuring anterior laminar depth.The present study also used this reference plane.However, a recent prospective observational study[24]found the anterior movement of the lamina was detected more frequently with the Bruch’s membrane compared with the anterior sclera reference plane.Signifcant choroidal thinning occurred in most patients in whom anterior movement of the lamina occurred with the Bruch’s membrane, but not the anterior sclera reference plane,indicating the decrease of anterior laminar depth measured based on the Bruch’s membrane may due to the thinning of choroids.In the present study, though the correlation between age and ALCSD didn’t reach statistical significance, the average ALCSD of the OG was smaller than that of YG and MG.Dinget al[25]found that the choroidal thickness reduced significantly in old people above 60y, the reduced choroidal thickness may result in reduced ALCSD measured in older group (age above 60y) in the present study.
A study[20]reported that the mean prelaminar tissue volume was lower in the older subjects compared to the younger subjects of European descent and African descent.In the present study, we also found that the cPLTT decreased with age.It is well-known that the ganglion cell axon and the vessels are two main components of prelaminar tissue[26].Studies[27]have demonstrated that the ganglion cell axon lost with age.Bazvandet al[28]also found that the flow area of the ONH and the papillary vascular density decreased with increasing age.Thus, the attenuation of ganglion cell axon and the blood flow volume may lead to the decrease of cPLTT.A study in rhesus monkeys with experimental glaucoma displayed that overall tissues were atrophied in the prelaminar region in glaucomatous eyes[22].Chunget al’s[18]study even found that the progressed glaucoma group had a signifcantly thinner prelaminar tissue than the non-progressed group at baseline, indicating that thinner prelaminar tissue may be a risk of glaucoma progression.The decrease in cPLTT with age may also prompt the increasing risk of glaucoma and be consistent with the investigation results that primary open angle glaucoma prevalence increased per decade of age[10].
The relationship between AL and cLCT was controversial in the previous studies.Leeet al’s[14]study revealed that AL was not correlated with cLCT, which was consistent with our results.However, a histologic study by Jonaset al[13]indicated that the lamina was significantly thinner in highly myopia.Since high myopic eyes are always accompanied by multiple fundus abnormalities, high myopia eyes (<-6 D) which always with an extremely long AL were not recruited in this study.The small range of AL might be one of the reasons that we could not fnd a relationship between AL and cLCT.
The present study revealed that the thickness of the cLCT and cPLTT might be related to age in healthy Chinese subjects.The impact of the age should be taken into account when evaluating the LC in glaucoma and other optic neuropathies.There are still several limitations in this study.First, in this study the cLCT, cALSCD and cPLTT were calculated from the average value of the LCT, ALCSD and PLLTT in the ONH central point and paracentral points (150 μm from the centre point) in the horizontal and vertical directions, which just is a preliminary study about the central LC and cannot refect the overall perspective of the LC.Further studies are needed to increase the scanning density of the lamina, reduce the shadow of the vessel in the peripheral laminar region and carry out three-dimensional reconstruction of the lamina in order to obtain a more comprehensive understanding of the lamina.Second, the LC curvature was not included in this study.Due to the shadow of the vessel in the peripheral laminar region,the peripheral laminar image is not clear enough to accurately calculate the laminar curvature, further study using sweptsource OCT which can provide imaging with better penetration may resolve this problem.Third, as the reference plane may affect the ALCSD measurement, a further study is needed to compare the influence of age on ALCSD using different measurement method.
ACKNOWLEDGEMENTS
Authors’ contributions:Hui Xiao: designing the study, analyzing the data and writing the manuscript; Xiao-Yu Xu: collecting and analyzing the data; Yi-Min Zhong: editing the important part of the paper; Xing Liu: editing the important part of the paper.
Foundation:Supported by Natural Science Foundation of Guangdong Province, China (No.2017A030313649).
Conflicts of Interest:Xiao H, None; Xu XY, None; Zhong YM, None; Liu X, None.
REFERENCES
1 Crawford Downs J, Roberts MD, Sigal IA.Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism.Exp Eye Res2011;93(2):133-140.
2 Beotra MR, Wang X, Tun TA, Zhang L, Baskaran M, Aung T,Strouthidis NG, Girard MJA.In vivo three-dimensional lamina cribrosa strains in healthy, ocular hypertensive, and glaucoma eyes following acute intraocular pressure elevation.Invest Ophthalmol Vis Sci2018;59(1):260-272.
3 Jonas JB, Wang NL, Wang YX, You QS, Xie XB, Yang DY, Xu L.Estimated trans-lamina cribrosa pressure difference versus intraocular pressure as biomarker for open-angle glaucoma.The Beijing Eye Study 2011.Acta Ophthalmol2015;93(1):e7-e13.
4 Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR.Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma.Am J Ophthalmol1983;95(5):673-691.
5 Levy NS, Crapps EE, Bonney RC.Displacement of the optic nerve head.Response to acute intraocular pressure elevation in primate eyes.Arch Ophthalmol1981;99(12):2166-2174.
6 Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF.3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness.Invest Ophthalmol Vis Sci2007;48(10):4597-4607.
7 Kotecha A, Izadi S, Jeffery G.Age-related changes in the thickness of the human lamina cribrosa.Br J Ophthalmol2006;90(12):1531-1534.
8 Park HY, Jeon SH, Park CK.Enhanced depth imaging detects lamina cribrosa thickness differences in normal tension glaucoma and primary open angle glaucoma.Ophthalmology2012;119(1):10-20.
9 Park SC, Brumm J, Furlanetto RL, Netto C, Liu Y, Tello C, Liebmann JM, Ritch R.Lamina cribrosa depth in different stages of glaucoma.Invest Ophthalmol Vis Sci2015;56(3):2059-2064.
10 Kapetanakis VV, Chan MP, Foster PJ, Cook DG, Owen CG, Rudnicka AR.Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis.Br J Ophthalmol2016;100(1):86-93.
11 Park SC, Ritch R.High resolution in vivo imaging of the lamina cribrosa.Saudi J Ophthalmol2011;25(4):363-372.
12 Ren R, Li B, Gao F, Li L, Xu X, Wang N, Jonas JB.Central corneal thickness, lamina cribrosa and peripapillary scleral histomorphometry in non-glaucomatous Chinese eyes.Graefes Arch Clin Exp Ophthalmol2010;248(11):1579-1585.
13 Jonas JB, Berenshtein E, Holbach L.Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fuid space.Invest Ophthalmol Vis Sci2003;44(12):5189-5195.
14 Lee EJ, Kim TW, Weinreb RN, Suh MH, Kim H.Lamina cribrosa thickness is not correlated with central corneal thickness or axial length in healthy eyes: central corneal thickness, axial length, and lamina cribrosa thickness.Graefes Arch Clin Exp Ophthalmol2013;251(3):847-854.
15 Albon J, Purslow PP, Karwatowski WS, Easty DL.Age related compliance of the lamina cribrosa in human eyes.Br J Ophthalmol2000;84(3):318-323.
16 Albon J, Karwatowski WS, Easty DL, Sims TJ, Duance VC.Age related changes in the non-collagenous components of the extracellular matrix of the human lamina cribrosa.Br J Ophthalmol2000;84(3):311-317.
17 Liu B, McNally S, Kilpatrick JI, Jarvis SP, O'Brien CJ.Aging and ocular tissue stiffness in glaucoma.Surv Ophthalmol2018;63(1):56-74.
18 Chung HS, Sung KR, Lee JY, Na JH.Lamina cribrosa-related parameters assessed by optical coherence tomography for prediction of future glaucoma progression.Curr Eye Res2016;41(6):806-813.
19 Sigal IA, Flanagan JG, Lathrop KL, Tertinegg I, Bilonick R.Human lamina cribrosa insertion and age.Invest Ophthalmol Vis Sci2012;53(11):6870-6879.
20 Rhodes LA, Huisingh C, Johnstone J, Fazio M, Smith B, Clark M,Downs JC, Owsley C, Girard MJ, Mari JM, Girkin C.Variation of laminar depth in normal eyes with age and race.Invest Ophthalmol Vis Sci2014;55(12):8123-8133.
21 Ren R, Yang H, Gardiner SK, Fortune B, Hardin C, Demirel S,Burgoyne CF.Anterior lamina cribrosa surface depth, age, and visual feld sensitivity in the Portland progression project.Invest Ophthalmol Vis Sci2014;55(3):1531-1539.
22 Hayreh SS, Pe’er J, Zimmerman MB.Morphologic changes in chronic high-pressure experimental glaucoma in rhesus monkeys.J Glaucoma1999;8(1):56-71.
23 Burgoyne CF, Downs JC.Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head.J Glaucoma2008;17(4):318-328.
24 Vianna JR, Lanoe VR, Quach J, Sharpe GP, Hutchison DM, Belliveau AC, Shuba LM, Nicolela MT, Chauhan BC.Serial changes in lamina cribrosa depth and neuroretinal parameters in glaucoma: impact of choroidal thickness.Ophthalmology2017;124(9):1392-1402.
25 Ding X, Li J, Zeng J, Ma W, Liu R, Li T, Yu S, Tang S.Choroidal thickness in healthy Chinese subjects.Invest Ophthalmol Vis Sci2011;52(13):9555-9560.
26 Park SC.In vivo evaluation of lamina cribrosa deformation in glaucoma.J Glaucoma2013;22 Suppl 5:S29-S31.
27 Zhang X, Francis BA, Dastiridou A, Chopra V, Tan O, Varma R,Greenfeld DS, Schuman JS, Huang D; Advanced Imaging for Glaucoma Study Group.Longitudinal and cross-sectional analyses of age effects on retinal nerve fber layer and ganglion cell complex thickness by fourierdomain OCT.Transl Vis Sci Technol2016;5(2):1.
28 Bazvand F, Mirshahi R, Fadakar K, Faghihi H, Sabour S, Ghassemi F.The quantitative measurements of vascular density and fow area of optic nerve head using optical coherence tomography angiography.J Glaucoma2017:26(8):735-741.
Citation:Xiao H, Xu XY, Zhong YM, Liu X.Age related changes of the central lamina cribrosa thickness, depth and prelaminar tissue in healthy Chinese subjects.Int J Ophthalmol2018;11(11):1842-1847
DOl:10.18240/ijo.2018.11.17
Accepted:2018-07-21
Received:2018-04-13
Correspondence to:Xing Liu.No.7, Jinsui Road, Tianhe District, Guangzhou 510060, Guangdong Province, China.drliuxing@163.com



