INTRODUCTION
Pericyte is vascular mural cell, embedded in membrane base and makes direct contact with endothelial. The highest pericyte coverage is observed in the retinal vasculature,which the ratio to endothelial is 1:1. The higher ratio of pericyte: endothelial, the more important function at the organ. It has been associated with vascular stabilization, like controlling blood flow, capillary permeability, and endothelial regulation[1-5]. Pericyte will lost in hyperglycemia condition.It is due to alteration of biochemical pathway, and growth factors. Because the retinal vascular appears to be the most sensitive site for pericyte loss, so the role of the pericyte in early diabetic retinopathy is very important.
Pericyte loss is the earliest morphological changes in the diabetic retina which will further affect instability of retinal vasculature. Few researcher have focused on the mechanism of pericyte loss, which is pericyte migration, but the mechanism is still unknown[6-7].
Angiopoietin-2 (Ang-2) is growth factor that increases in several conditions, like hyperglicemia. Angiopoietin regulates two pathways that mediated cellular motility through its receptor, Tie-2, with activate phosphotidylinositol 3 kinase(PI3K) and through mitogen-activated protein kinase (MAPK)pathways. The role of Ang-2 in Tie-2 phosphorylation is complicated that depends on the stimuli and the cells[8-10]. The exact role of Ang-2 induced Tie-2 phosphorylation in pericyte and the mechanism of pericyte migration due to hyperglicemia still poorly understood. The aim of this study is to investigate the molecular mechanism of pericyte migration in hyperglicemic retina observed from Ang-2/Tie-2 phosphorylation, Akt/PKB phosphorylation, and ERK1/2 phosphorylation in vivo.
MATERIALS AND METHODS
All experiments in this study were performed with approval from Ethics Committee Medical Faculty Brawijaya University.Animal were housed in groups in cages with standard food and water under 12h light and 12h dark rhythm. Rats were divided into 5 groups. Group 1 was normoglycemic rats (negative control), Group 2-5 were diabetic rats groups. Group 2 was group with DMSO intravitreal injection (positive control),Group 3 was group with Tie-2 inhibitor injection, Group 4 was inhibited with ERK1/2 intravitreal injection, and the last was Akt/PKB inhibition. Ang-2 serum was taken from each group before sacrificed.
Induction of Diabetes Mellitus Streptozotocin (STZ) was given 40 mg/kg·d for 5d for inducing diabetic rats. STZ injections were given intraperitoneally and the doses were determined according to the body weight of animals. The blood glucose concentration was measured every week from the day of STZ injection. STZ-injected animals were considered diabetic when blood glucose levels reached stable levels (>250 mg/dL).Diabetic and nondiabetic rats were sacrificed 5wk afterdiabetes induction, and eyes were enucleated under deep anesthesia and immediately frozen and fixed with 10% formalin.
Retinal Digest Preparation Retinal digest preparation of each groups were performed using trypsin 3% and done for three days in order to make separation of retinal vasculature to another eye substance like previously described[11]. Each retinal digest eye divided into 5 groups, for examining pericyte and endothelial morphology [stained with hematoxylineasin (HE)], and the rest for analyzed the phosphorylation of each migration pathway according to antibody that given to retinal digest preparation [anti-phospho Tie-2/TEK (Tyr 992)antibody, EMP Milipore corp, USA], phospho ERK1/2 [antiphospho ERK1/2 phospospecific-p44/42 MAPK (ERK1/2)(thr 202/Tyr204) cell signaling tech], and phosphoPkb antiphospho PKB (pSer 473), Sigma-aldrich].
Morphological Quantification We analyzed retinal digest preparation to count the pericyte: endothelial ratio. We counted it based on pericyte morphology and location site (branch,straight capillary, migration pericyte). Migrating pericyte defined as pericytes with triangular nuclei, migrating from capillaries into the extravascular interstitium, since at least one lateral side of the triangular nuclei was longer than the basis in contact to the capillary. Total number of pericyte were count on 3 random area (400× magnification) using image analyzing system (BX-53; Olympus Opticals, Hamburg, Germany).
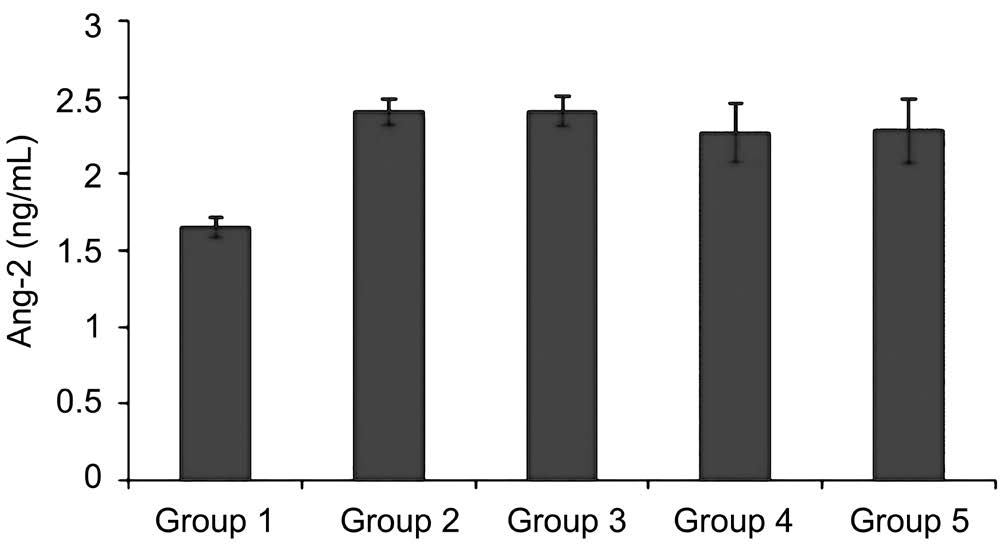
Figure 1 Ang-2 level on diabetic rat models There were significant differences between normoglycemic rat (Group 1) and diabetic rats(Groups 2-5) (P<0.05).
Intravitreal Injection of Tie-2 Inhibitor, ERK1/2 Inhibitor,Akt/PKB Inhibitor and DMSO After the rats anesthetized with ketamine hydrochloride intraperitoneally and pontocaine eyedrop for eyes anesthesia, we gave intravitreal injection of sterilized solution of Tie-2 inhibitor (CAS 948557-43-5, santacruz biotech Inc.), 0.1 mm in phosphate buffer saline (PBS) with a concentration of 5% of dimethyl sulfoxide (DMSO)/5 μL. We performed intravitreal injection of ERK1/2 inhibitor (U0126,santacruz biotech), and Akt/PKB inhibitor (API-1, Torics,bioscience) with the same dose. Intravitreal injection were given 1wk after diabetes, under operating microscope (DECA-21, INAMI). After the injection we give antibiotics eyedrop(Tobrex, Alcon).
Phosphorylation of Tie-2, ERK1/2 and Akt/PKB From retinal digest preparation we used double staining methods,with primary antibody NG2 with rhodamin and FITC. NG2 is the specific marker for pericyte. Each groups coated with phospho Tie-2 [anti-phospho Tie-2/TEK (Tyr 992) antibody,EMP Milipore corp, USA], phospho ERK1/2 [anti-phospho ERK1/2 phosphospecific-p44/42 MAPK (ERK1/2) (thr 202/Tyr204) cell signaling tech], dan phospho PKB [anti-phospho PKB (pSer 473), Sigma-aldrich]. Analyzing using confocal laser scanning microscope FV1000 (Olympuss, USA).
Statistical Analysis Quantitative data were given as mean±SD.Linear regression test was used to make correlation between two variable. A value of P<0.05 was considered statistically significant.
RESULTS
Diabetic rats developed diabetes after 2wk. Ang-2 serum were significantly elevated in all diabetic rats groups compared with normoglycemic Group (P<0.05) (Figure 1). To assess the correlation between pericyte migration with increasing of Ang-2, we counted the number of pericyte and endothelial ratio from retinal digest preparation, and compared the number of pericyte migration with total number of pericyte on each groups. From linear regression we found correlation between Ang-2 and the number of pericyte migration (Figure 2). The type of pericyte can be seen on Figure 3.
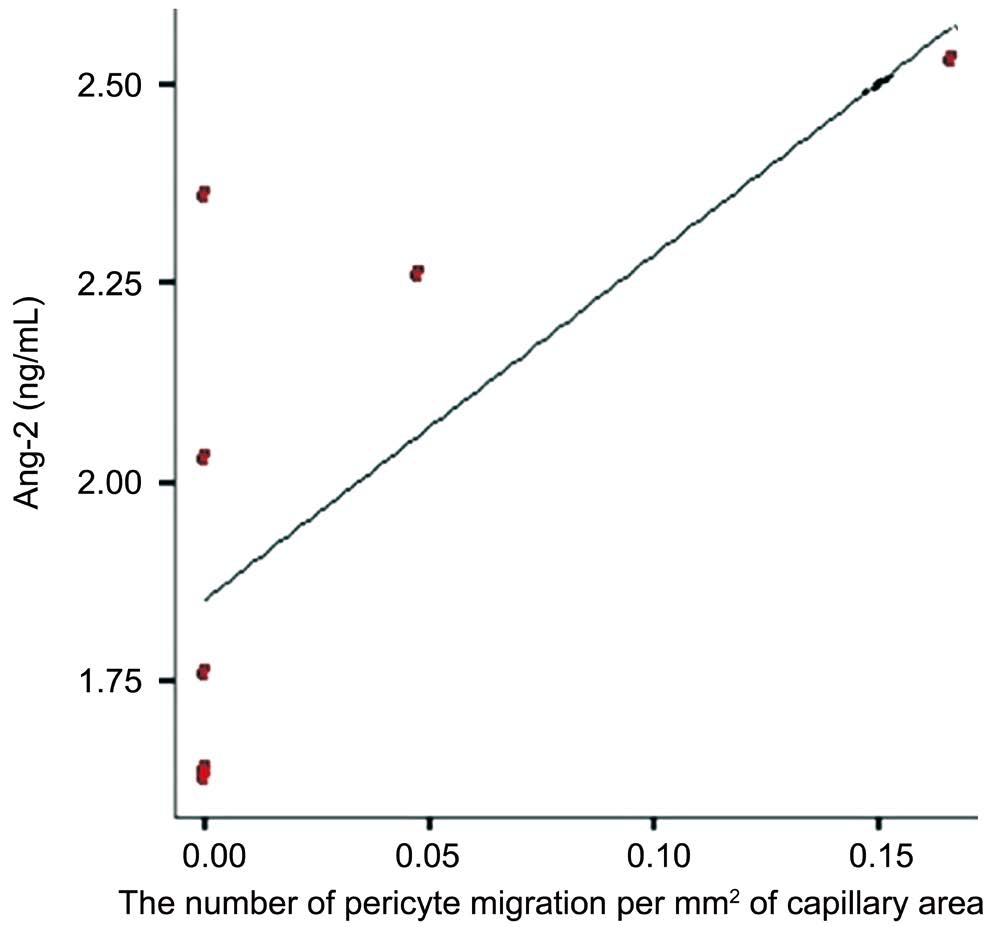
Figure 2 Ang-2 level correlated with the number of pericyte migration There were significant positive correlation between level of Ang-2 to pericyte migration (P<0.05).
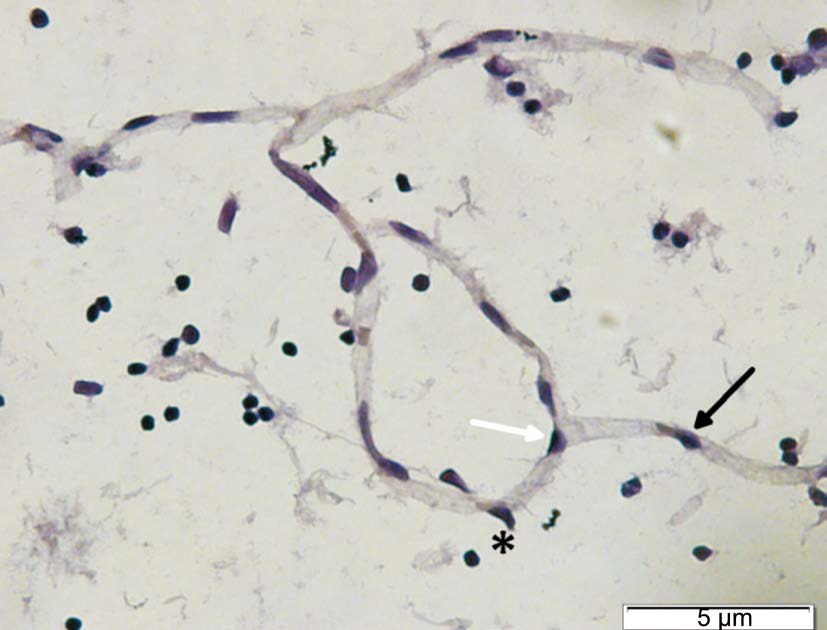
Figure 3 The illustration of pericyte Pericyte on straight capillaries(black arrow), pericyte on branch (white arrow) and pericyte migration(asterisk).
The differential between normal and diabetic rat retinal vasculature has already seen after 5wk suffer from diabetes(Figure 4A, 4B). From each groups we found that the ratio of pericyte: endothelial decrease in positive control group(1:2.4) compared with negative control group (1:1.2). There was differential pericyte:endothelial ratio on groups with inhibition in migration pathway. On groups that given Tie-2 intravitreal inhibitor we found pericyte: endothelial ratio of 1:1.8. The same result shows on group with Akt/PKB inhibition. ERK1/2 inhibitor group shows the best results of pericyte: endothelial ratio (1:1.7). From all diabetic rats groups the most pericyte migration found on Tie-2 inhibitor groups(0.14) (Group 3), and the least pericyte migration found on ERK1/2 inhibitor groups (0.025) (Group 4).
The mechanism of pericyte migration can be seen by evaluating the phosphorylation of Tie-2, ERK1/2 and Akt/PKB of the pericyte on each group using confocal microscope. In Figure 5 we can see there is phosphorylation of Tie-2, ERK1/2,and Akt/PKB on the negative control pericyte.
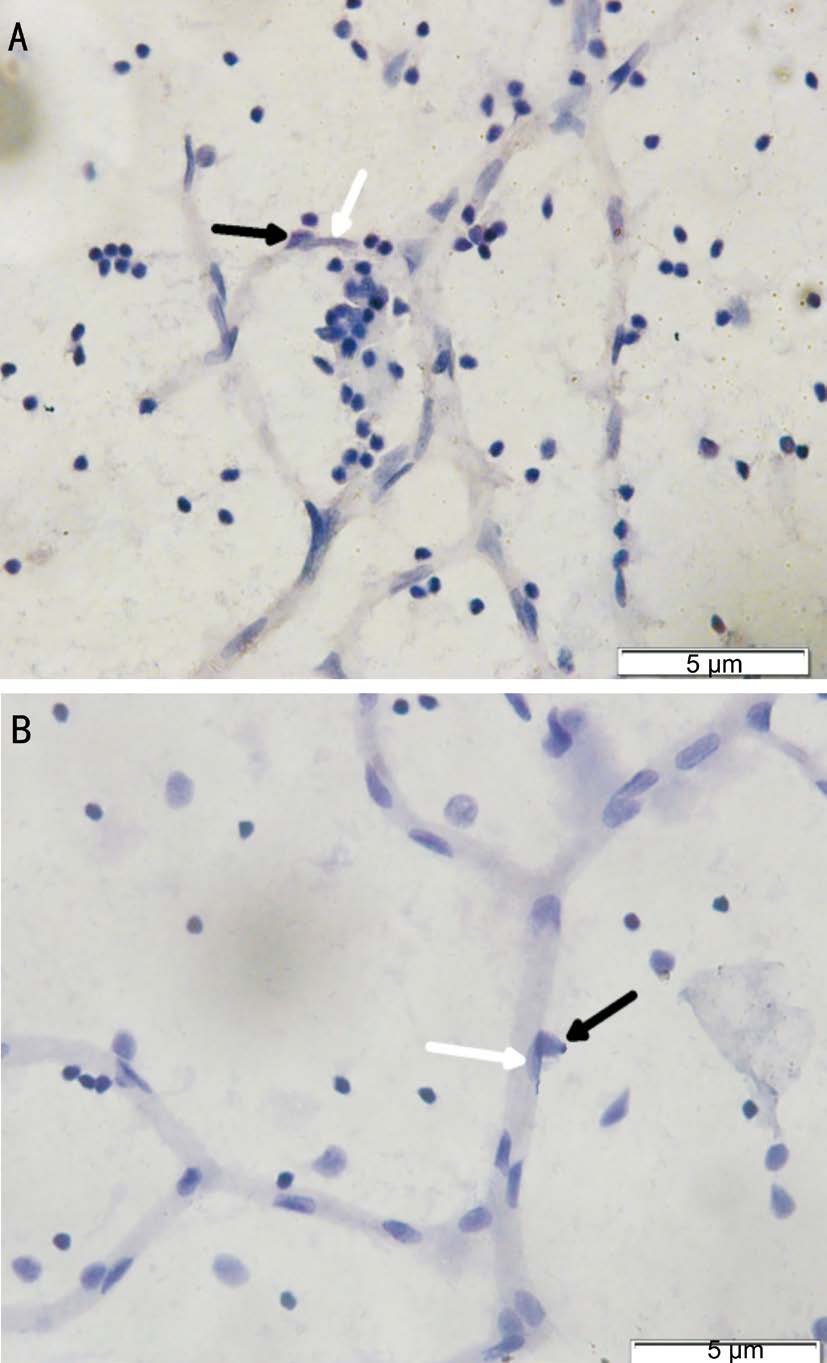
Figure 4 The retinal vasculature on normoglycemic rat (A) and diabetic rat (B) The vascular looks more dilated on diabetic rats, and the presence of pericyte less than normal retina. Pericyte was darker and round shape (black arrow), endothelial was lighter and spindle shape (white arrow). Original magnification: 600×.
The result of quatitative measurement of pericyte phosphorylation of each group shows that effect of Tie-2 inhibitor decreasing activity of Tie-2 phosphorylation, and affect downstream signaling pathway, ERK1/2 and Akt/PKB. ERK1/2 inhibitor decrease phosphorylation on Tie-2 receptor and affect Akt/PKB. But inhibition of Akt/PKB shows that the phosphorylation of Tie-2 is relatively the same compared to positive control group, and the phosphorylation of ERK1/2 were slightly decreased (Figure 6). The confocal microscope image of phosphorylation of Tie-2, ERK1/2, and Akt/PKB on the pericyte in negative control group shown on Figure 5, and the phosphorylation in positive control group and inhibitor groups can be seen in Figure 7.
DISCUSSION
Pericyte have been shown to have an important role in retinal vascular stabilization. The normal ratio of pericyte and endothelial covered retinal microvascular is 41%-50% (1:1). It is because the retinal blood flow have the highest metabolism of the body, so retinal tissue needs the tight vascular. The more pericyte covers vascular, the higher function as a barrier. As an endothelial barrier, the loss of pericyte may lead to alteration of endothelial behavior, and change the vascular permeability[1-4].Pericyte loss is the earliest histological change and the characteristic of diabetic retina. Previous study showed that Ang-2 will increase on hyperglycemic condition. This study proves that on all diabetic rats groups, there were significant increases of Ang-2 (P<0.05) compared to normoglycemic rats(1.66 ng/mL). According to previous study, hyperglycemia induce ROS overproduction from mitochondria, than produce modified gene transcription, including transcription of Ang-2[12-13]. According to Yao et al[14]the increasing of Ang-2 caused by methylglyoxal modification, and subsequently decreasing bonding to glucose-responsive GC box at Ang-2 promoter,than increasing Ang-2 transcription.
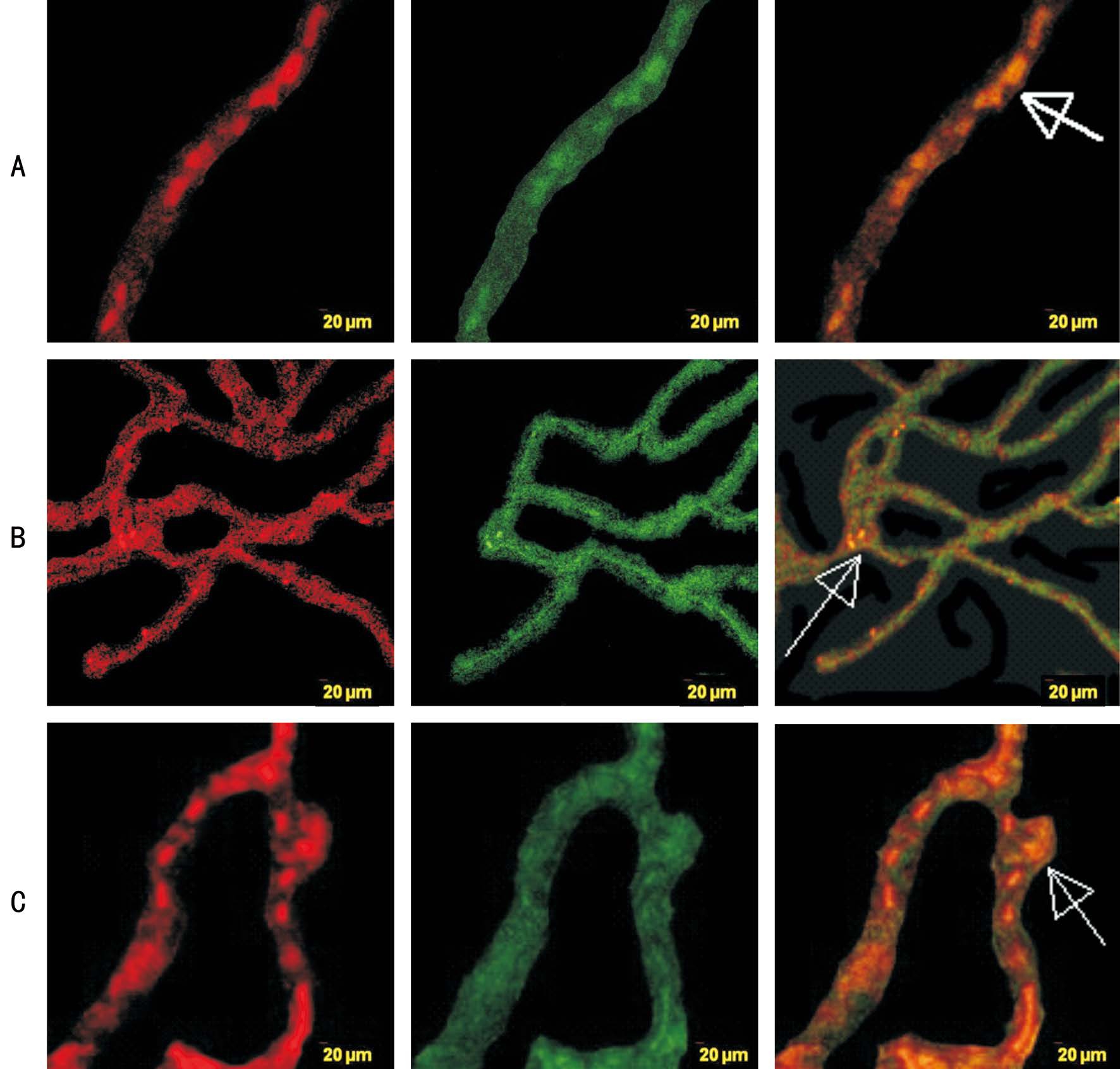
Figure 5 Pericyte identification in negative control groups with Tie-2, ERK1/2, and Akt/PKB phosphorylation A: Phospho Tie-2 antibody were given; B: Phospho ERK1/2 antibody were given; C: Akt/PKB antibody were given; NG-2-rhodamin (red) staining for pericyte identification, phospho antibody-FITC (green) staining for phosphorylated antibody, Rhodamin and FITC merge (orange) for identifying pericyte phosphorylated antibody (white arrow). Original magnification: 400×.
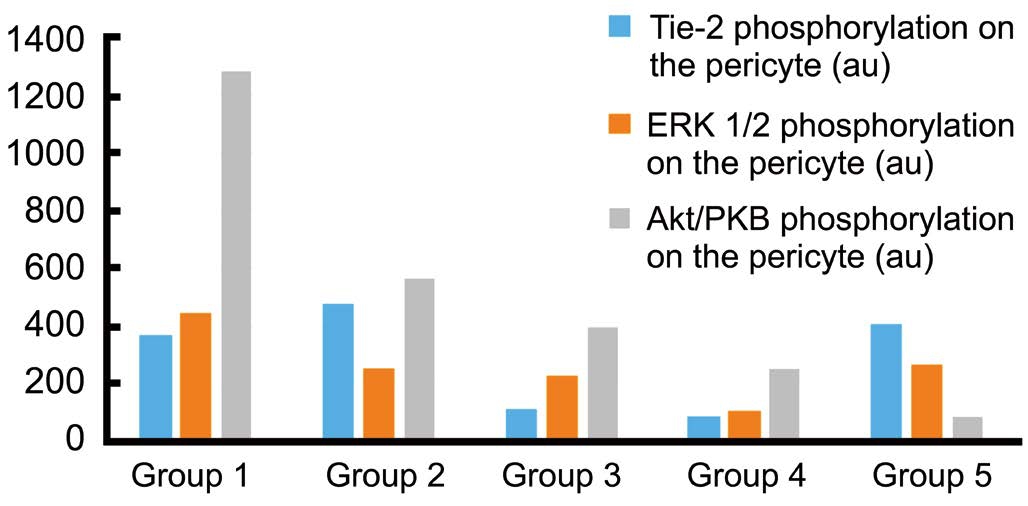
Figure 6 Tie-2, ERK1/2, and Akt/PKB phosphorylation on the pericyte Group 1 was normoclycemic rat; Group 2 was diabetic rat injected with DMSO; Group 3 injected with Tie-2 inhibitor; Group 4 injected with ERK1/2 inhibitor, and Group 5 was injected with Akt/PKB inhibitor. The Tie-2 phosphorylation was decrease on group with Tie-2 inhibitor and ERK1/2 inhibitor; There were decreasing of ERK1/2 phosphorylation on group injected with Tie-2, ERK1/2, and Akt/PKB inhibitor; Decreasing of Akt/PKB phosphorylation shown on three group injected with Tie-2, ERK1/2, and Akt/PKB inhibitor.Quantitative measurement using confocal microscope (au).
Angopoietin produced by Weibel-Palade bodies of endothelial has roles for vessel destabilization if there were specific stimuli. It produces on many condition like cancer, chronic disease, metabolic disease, and sepsis[15-16]. The increasing Ang-2 level cause the persistent disruption of cross-talk between pericyte and endothelial, then the pericyte is loss and retinal vessel destabilized[6]. At hyperglycemic condition, it makes thefirst characteristic of diabetic retinopathy.

Figure 7 Confocal microscope images of phosphorylation of Tie-2, ERK1/2, and Akt/PKB in positive control group (diabetic rat),and inhibitor groups Illustration of Tie-2, ERK1/2, and Akt/PKB phosphorylation on diabetic rat injected with DMSO intravitreal (A-C);Phosphorylation of Tie-2, ERK1/2, and Akt/PKB on diabetic rat pericyte injected with Tie-2 inhibitor intravitreal (D-F); Phosphorylation of Tie-2,ERK1/2, and Akt/PKB on diabetic rat pericyte injected with ERK1/2 inhibitor intravitreal (G-I); Phosphorylation of Tie-2, ERK1/2, and Akt/PKB on diabetic rat pericyte injected with Akt/PKB inhibitor intravitreal (J-L). Original magnification: 400×.
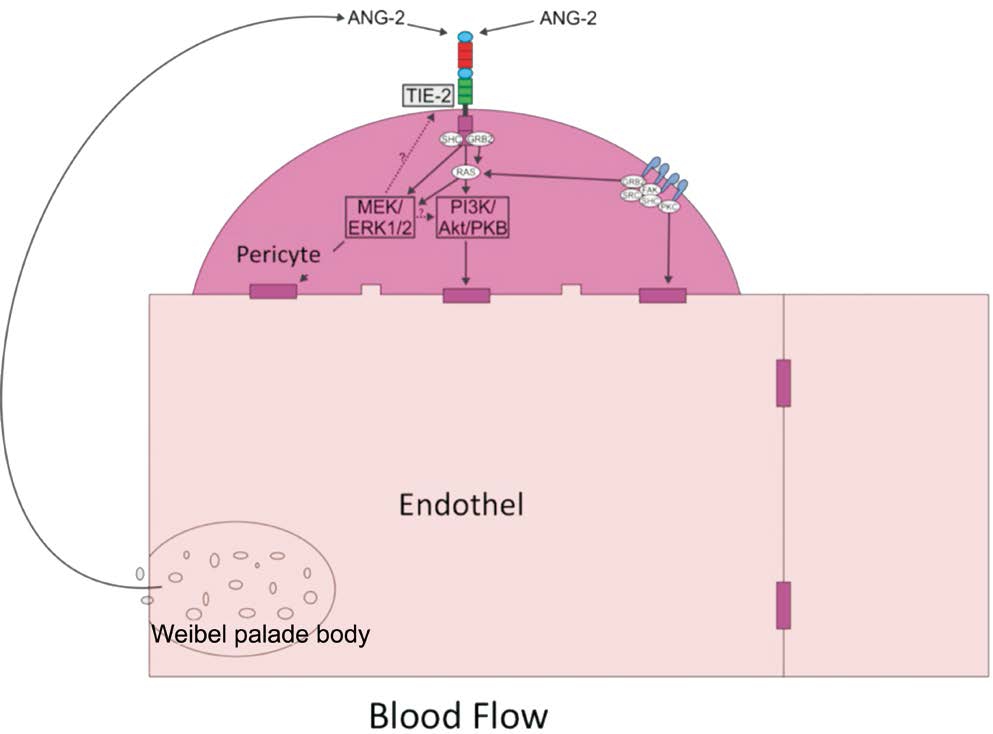
Figure 8 Mechanisme of pericyte migration through Ang-2/Tie-2, ERK1/2,and Akt/PKB signaling pathway Tie-2 reseptor phosphorylated by Ang-2 producted from Weibel-Pallade bodies; Tie-2 phosphorylation in fluence the downstream pathways (ERK1/2 and Akt/PKB).
Recent study have showed that Ang-2 induce pericyte loss through migration process, but the mechanism of pericyte migration in hyperglycemic state is unclear[7]. Angiopoietin regulates two pathways that mediated cellular motility through its receptor, Tie-2, with activate PI3K and through MAPK pathways. Many study investigate the mechanism of migration pathway through those major signalling pathway but the result still controversial[17-20].
The role of Ang-2 in Tie-2 phosphorylation is complicated,depend on the stimuli and the cells. The exact role of Ang-2 induced Tie-2 phosphorylation in pericyte and the mechanism of pericyte migration due to hyperglicemia still unknown.In this study we try to explain the mechanism of pericyte migration, through inhibition of migration pathway.
On the diabetic rat models group received Tie-2 inhibitor intravitreal, the pericyte loss and migration still happened.Although Ang-2 has been postulated to naturally bind to the Tie-2 receptor in the pericyte as the Ang-Tie system, but theoretically Ang-2 can directly bind integrin, FAK, and subsequently affect downstream signaling pathway, like ERK1/2 and Akt/PKB[17-18, 21-22]. According to Park et al[22]there were no Tie-2 receptor in human pericyte. This statement is conflicting with previous study[7,22-23], and with the result on this study.
Even though the pericyte loss cannot be stopped maximally,but the decreasing of ERK1/2 and Akt/PKB phosphorylation sugest that inhibition of Tie-2 phosphorylation still have a role for pericyte migration pathway (Figure 8). It described from the decreasing of pericyte loss which is better at the hypergliclemic rat injected with Tie-2 inhibitor intravitreal(1:1.8) than those in positive control group (1:2.4).
Inhibition with ERK1/2 inhibitor decrease activity of all migration pathway examined in this study. We assumed there were feedback mechanism that makes the upstream pathway(Tie-2) decreased than in fluences the decreasing of Akt/PKB phosphorylation. Other possibilities shows there is intracellular crosstalk between ERK1/2 and Akt/PKB without involvement of Tie-2 receptor, so the phosphorylation of Akt/PKB decresing with ERK1/2 inhibitor intravitreal. The results showed that the pericyte: endothelial ratio of groups injected with ERK1/2 inhibitor (1:1.7) is the best compared to another diabetic groups in this study.
On the diabetic rat model received Akt/PKB inhibitor intravitreal, we found a dramatically decrease of Akt/PKB phosphorylation, but the changes of Tie-2 and ERK1/2 phosphorylation were minimal. We assume Akt/PKB pathway involed in pericyte migration based on the decreasing of its phosphorylation after administered with Akt/PKB inhibitor,and the ratio of pericyte endothel is better than positive control group (1:1.8 vs 1:2.4).
There is supposition that pericyte migration caused by lack of nutritions and environment stress at vascular site, so the pericyte migrate to perivascular. Although pericyte does not contact to blood flow directly, but hypoxia due to hemoreological changes of diabetes makes environmental stress that leading to pericyte migration. Deformity changes and erythrocyte aggregation(rolleaux formation) due to dibetes has a role of increasing blood viscosity than lead to hemoreological changes[24-26].
According to previous study pericyte apoptosis have been detected after 6mo of experimental diabetes, but our study shows that pericyte loss already detectable after 5wk[7]. We still do not know whether the lost of pericyte happened due to migration only or there was apoptotic process accompany the migration process. Dramatically decreasing Akt/PKB phosphorylation indicate that it is possible that apoptotic process has occured, because one of the Akt/PKB functions is for cellular stability.
Unfortunately in this study we did not examine the apoptotic parameters and dose dependency for inhibition migration pathway. For further research we can investigate the pericyte migration pathway in dose dependency of inhibitors.
The ERK1/2 pathway seems most important pathway for pericyte migration. On diabetic rats, inhibition of ERK1/2 pathway decrease Tie-2 through upstream signalling pathway and decrease activity of Akt/PKB. The number of pericyte migration is the smallest of all diabetic rats groups, and the pericyte: endothelial ratio is closer to normoglycemic rat group. Targeting ERK1/2 pathway can be a novel therapeutic strategy for prevention of diabetic retinopathy.
ACKNOWLEDGEMENTS
conflicts of Interest:Dewi NA, None; Aulanni'am A, None;Sujuti H, None; Widodo MA, None; Soeatmadji DW, None.
REFERENCES
1 Shepro D, Morel NM. Pericyte physiology. FASEB J 1993;7(11):1031-1038.
2 Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs (Print) 2001;169(1):1-11.
3 Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions.Circ Res 2005;97(6):512-523.
4 Pfister F, Feng Y, Hammes HP. Pericyte loss in the diabetic retina.Diabetic Retinopathy 2008;245-264.
5 Anand-Apte B, Hollyfield JG, Developmental anatomy of the retinal and choroidal vasculature. Encyclopedia of the Eye 2010;9-15.
6 Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci 2008;49(5):2163-2171.
7 Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 2008;57(9):2495-2502.
8 Thurston G, Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb Perspect Med 2012;2(9):a006550.
9 Bogdanovic E, Nguyen VP, Dumont DJ. Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J Cell Sci 2006;119(Pt 17):3551-3560.
10 Welf ES, Haugh JM. Signaling pathways that control cell migration:models and analysis. Wiley Interdiscip Rev Syst Biol Med 2011;3(2):231-240.
11 Dewi NA, Haq NMD, Sujuti H, Aulanni’am A, Soeatmadji DW.Retinal digest procedures for examining pericyte and endothelial on retinal vasculature using rat model. International Journal of Chemtech Research 2015;8(6):607-611.
12 Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T,Renner O, Breier G, Brownlee M, Deutsch U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 2004;53(4):1104-1110.
13 Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy:targeting vasoregression. Diabetes 2011;60(1):9-16.
14 Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I,Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 2007;282(42):31038-31045.
15 Ghazaly A, Moes A, Shorbagy M, Nahrery E. Angiopietin-2 as biomarker of metabolic syndrome and disease activity in rheumatoid arthrtitis patients. The Egyptian Rheumatologist 2015;38(1):9-13.
16 Kümpers P, van Meurs M, David S, Molema G, Bijzet J, Lukasz A, Biertz F, Haller H, Zijlstra JG. Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Crit Care 2009;13(3):R64.
17 Ahmad S, Cudmore MJ, Wang K, Hewett P, Potluri R, Fujisawa T,Ahmed A. Angiopoietin-1 induces migration of monocytes in a tie-2 and integrin-independent manner. Hypertension 2010;56(3):477-483.
18 Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S,Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 2012;122(6):1991-2005.
19 Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration.Circ Res 2007;100(5):607-621.
20 Li L, Wang Z, Jiang J, Zao M. Signaling pathways involved in migration of mesenchymal stem cells. Trends in Bio/Pharmaceuticall Industry 2010;(6):29-33.
21 Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell 2006;98(9):547-555.
22 Park SW, Yun JH, Kim JH. Kim KW, Cho CH, Kim JH Angiopoietin 2 induces pericyte apoptosis via α3β1 integrin signaling in diabetic retinopathy. Diabetes 2014;63(9):3057-3068.
23 Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A,Schlereth K, Augustin HG. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun 2017;8:16106.
24 Czaja M, Kabat M, Wasilewski J. Hemorheological parameters and fibrinogen in atherosclerosis. Journal of Cardiology and Therapy 2014;1(9).
25 Irace C, Carallo C, Scavelli F, De Franceschi MS, Esposito T, Gnasso A. Blood viscosity in subjects with normoglycemia and prediabetes.Diabetes Care 2014;37(2):488-492.
26 Yeom E, Byeon H, Lee SJ. Effect of diabetic duration on hemorheological properties and platelet aggregation in streptozotocininduced diabetic rats. Scientific Reports 2016;6(1).