Production of interleukin-1β related to mammalian target of rapamycin/Toll-like receptor 4 signaling pathway during As pergillus fumigatus infection of the mouse cornea
Rui Xu, Jing Lin, Gui-Qiu Zhao, Cui Li, Cheng-Ye Che, Qiang Xu, Min Liu
Department of Ophthalmology, the Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong Province, China
Abstract· AlM: To elucidate the effect of rapamycin on regulating the production of inter leukin (lL)-1β in Asper gillus fumig atus(A. fumigatus)-induced keratitis and to verify whether the expression of lL-1β in A. fumigatus keratitis is associated with the mammali an target of rapamycin (mTOR)/Toll-like receptor 4 (TLR4) signaling pathway.· METHODS: Fungal keratitis mouse models of susceptible C57BL/6 mice were established using A. fumi gatus. The mice were subsequently treated with rapamycin. The protein levels of p-mTOR, T LR4, and lL-1β in normal and infected corneal tissue were measured by Western blot. The TLR4 and lL-1β mRNA levels were determined by real-time polymerase chain reaction (PCR).· RESULTS: ln C57BL/6 mice, rapamycin treatment decreased the clinical scores and production of the pro-inflammatory cytokine, lL-1β. The expression of TLR4, stimulated by A.fumigatus, was reduced as well when the mTOR signaling pathway was suppressed by rapamycin.· CONCLUSlON: Rapamycin is beneficial for the outcome of fungal keratitis and has an inhibitory effect expression of the inflammatory cytokine lL-1β. The inhibitory effect on lL-1β expression can be associated with the mTOR/TLR4 signaling pathway in A. fumigatus infection in mice.
KEYWORDS: keratitis; interleukin-1β; mammalian target of rapamycin; Toll-like receptor 4; mice
INTRODUCTION
Fungal keratitis is a serious ocular disease with a high probability of causing blindness[1-2]. Pathogen-associated molecular patterns (PAMPs), comprising molecular structures unique to microbes, trigger the activation of inflammatory pattern recognition receptors (PRRs), such as Toll-like receptor (TLR), thus, leading to inflammatory phagocytosis and induction of immune responses[3-4]. Toll-likerecep tor 4(TLR4), which belongs to the TLR family of PRRs, plays a significant role in activating inflammatory signaling pathways in response to microbial pathogens[5-6], and studies have shown that TLR4 plays an important role in fungal keratitis in mice infected with Aspergillus fumigatus (A. fumigatus)[7-9].
Mammalian target of rapamycin (mTOR) consists of two distinct complexes called mammalian target of rapamycin complex 1 (mTORC1) and mammalian target of rapamycin complex 2 (mTORC2), and mTOR signaling impacts downstream cellular processes, including the cell cycle,cell growth, differentiation, survival, and metabolism[10-15].Rapamycin, a classical mTOR inhibitor[16], was discovered in soil samples from Easter Island in the 1970s and was found to have antifungal activity. Over the past few years, the suppressive effect of rapamycin on T cell activation, as part of adaptive immunity, has been investigated in depth, and clinically rapamycin has been used as an immunosuppressant in allogeneic transplantation. In addition, mTOR has been targeted in the treatment of various types of cancers[17-20].Now, it is increasingly clear that the mTOR signaling network plays an extremely important role in regulating the functions of innate immune cell populations and may further affect the outcome of infectious diseases[21-26]. Some researchers have reported that the inhibition of mTOR signaling on rapamycin treatment reduced the expression of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-2, IL-6, IL-10, IL-12,and tumor necrosis factor (TNF)-α in vivo or in vitro[16,27-30].However, the role of mTOR in innate immunity during fungal keratitis has not been reported.
IL-1β is one of the key cytokines involved in antifungal immunity[9,31-32]. TLR4 mediates the production of IL-1β in protective immunity[9,33-34]. Recently, studies have provided evidence that rapamycin suppresses TLR4-triggered IL-6 production in colon tumor cells by inhibiting TLR4 expression and nuclear factor-κB (NF -κB) activation. Rapamycin also reduced lipopolysaccharide (LPS)-induced IL-6 production in head and neck squamous cell carcinoma cells, and this effect was mediated by TLR4[35-37]. Rapamycin may attenuate diabetic nephropathy by suppressing TLR4 signaling and Th17 cell signaling. These findings suggest that the effects mediated by TLR4 are closely correlated with mTOR; however, whether IL-1β production could be regulated through the mTOR/TLR4 signaling pathway in the innate immune response during fungal keratitis has not yet been reported.
In the present study, we investigated whether mTOR participates in the regulation of inflammatory responses via the TLR4 signaling pathway. Our results show that after inhibiting phosphorylated mTOR (p-mTOR), the expression of TLR4 and IL-1β stimulated by A. fumigatus was suppressed during the innate immune response to fungal keratitis. These results suggest a potential role of mTOR in mediating IL-1β signaling,which may occur through the mTOR/TLR4 pathway during A. fumigatus infection in mice. These data may contribute to better understanding of fungal keratitis pathogenesis and provide new avenues for its treatment.
MATERIALS AND METHODS
Mice and Corneal Infection Specific pathogen-free(SPF) 8-week-old female C57BL/6 (susceptible) mice were purchased from Changzhou Cavens Laboratory Animal Co.,Ltd. (Changzhou, Jiangsu Province, China) and were carefully housed in a 22℃±2℃ environment. Their physiological condition was observed every day before and after A. fumigatus infection. The mice were anesthetized wit h 8% chloral hydrate.The mice were observed using a stereoscopic microscope at a magnification of 40×, and a scratch area with a radius of 2 mm was made on the central corneal epithelium. A 5 μL A. fumigatusinoculum (strain 3.0772, General Microbiological Culture Collection Center, Beijing, China), with a concentration of 1×108 CFU/mL, was applied on the damaged corneal surface. The cornea was covered with a soft contact lens before the eyelids were sutured. Mice corneal tissues was harvested for real-time reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot at 1 and 3d after infection. All treatments on mice were in accordance with the guidelines of the Chinese Ministry of Science and Technology Guidelines on the Humane Treatment of Laboratory Animals(vGKFCZ-2006-398) and the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Rapamycin Treatment Rapamycin (MCE, New Jersey, USA)was dissolved to a concentration of 20 μg/μL in 100% ethanol and stored at -20℃. Before subconjunctival and intraperitoneal injection, the rapamycin solution in ethanol was diluted with sterile phosphate buffer saline (PBS) containing 5% Tween-80. To determine the appropriate concentration of rapamycin, it was administered via subconjunctival injection into the left eye at a concentration of 0.1 μg/5 μL and 0.5 μg/5 μL in the preliminary experiments. Results indicated that the concentration of 0.5 μg/5 μL had significantly lower p-mTOR protein levels in the cornea before and after infection (comparative data not shown); thus, all subsequent experiments were performed using this concentration of rapamycin. In addition,in others studies using rapamycin on the eyes[38-39], researchers chose to pretreat mice with rapamycin intraperitoneally at a concentration of 6.0 mg/kg, so an additional 6.0 mg/kg was injected intraperitoneally on the day of infection and day 1 after infection in our experiment, as well. Results showed that this method was effective in inhibiting p-mTOR expression and IL-1β production. The vehicle group was given PBS containing 3%alcohol and 5% Tween-80. Corneal tissue was harvested for the further research.
Ocular Response to Aspergillus Fumigatus Infection The severity of keratitis in mice was scored visually, according to photographs taken using a slit-lamp. The clinical scoring criteria were as follows[40]: 0=no or slightly visible ulcer area;1=mild ulcer and anterior segment was covered partially;2=moderate ulcer and the pupil was partially or completely covered; 3=cornea was opaque and the complete anterior segment was covered; and 4=corneal perforation. The scores were recorded on day 1 post infection (p.i.) for each infected mouse before harvesting the cornea. Representative corneal tissue was photographed to document the effects of rapamycin vs vehicle treatment.
Real-time Reverse Transcriptase Polymerase Chain Reaction Normal (untreated; n=6/group/time), vehicle(injected with sterile PBS containing 3% alcohol and 5%Tween-80), and rapamycin-treated corneal tissue was harvested for detection of mTOR, TLR4, and IL-1β mRNA levels. cDNA was synthesized by reverse transcription of 2 μg total RNA using the PrimeScript RT Reagent Kit (Takara, Tokyo, Japan) and was diluted to 1:25 with diethylpyrocarbonate (DEPC)-treated water. Quantitative RT-PCR was performed using Eppendorf Mastercycler and SYBR green. The housekeeping gene, β-actin, served as the internal control. The oligonucleotide primers used were as follows: β-actin: F-GATTACTGCTCTGGCTCCTAGC,R-GACTCATCGTACTCCTGCTTGC; TLR4: F-CCTGACAC CAGGAAGCTTGAA, R-TCTGATCCATGCATTGGTAGGT;mTOR: F-ACCGGCACACATTTGAAGAAG, R-CTCGTT GAGGATCAGCAAGG; and IL-1β: F-CGCAGCAGCACAT CAACAAGAGC, R-TGTCCTCATCCTGGAAGGTCCACG.Each experiment was repeated at least three times.
Western Blot Corneal tissue was lysed in 200 μL r adioimmunoprecipitation assay (RI PA; Solarbio, Beijing,China) lysis buffer containing 2 μL phenyl methanes ulfonyl fluoride (PMSF; 100:1; Solarbio) and 2 μL protease inhibitor cocktail (100:1; MCE, Monmouth Junction, NJ, USA) for 2h. The samples were then centrifuged, SDS sample buffer was added, and the samples were boiled after determining the protein concentration. Total proteins were separated on 8%-16% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (Merck,Darmstadt, Germany). After blocking with Western blocking buffer (Beyotime, Jiangsu Province, China) at room temperature for 3h, the membranes were incubated overnight at 4℃ with a polyclonal antibody against β-actin (1:7000;Bioss, Beijing, China), prima ry antibody against p-mTOR(1:1000; Ser2448; Cell Signaling Technology, Danvers,MA, USA), monoclonal antibody against mTOR (1:1000;Abcam, Cambridge, UK), primary antibody again st TLR4(1:1000; Proteintech, Wuhan, China), or primary antibody against IL-1β (1:500; Bioss). The membranes were washed in PBS containin g 0.05% Tween-20 (Bio-Rad, Hercules, CA,USA) 3 times every 5min. Membranes were incubated with corresponding peroxidase-conjugated secondary antibodies(1:10 000; Abcam) at room temperature for 2h. Finally, the blots were visualized by a chemiluminescence system (ECL;Thermo Fisher Scientific, Waltham, MA, USA), and the protein levels were measured and normalized to the l evels of β-actin using ImageJ software.
Statistical Analysis Data were expressed as mean±standard deviation (SD) and were analyzed by GraphPad Prism 5.0 software. Statistical significance of real-time RT-PCR and Western blot data was determined by unpaired two-tailed Student’s t-test. Differences were considered significant at P≤0.05.
RESULTS
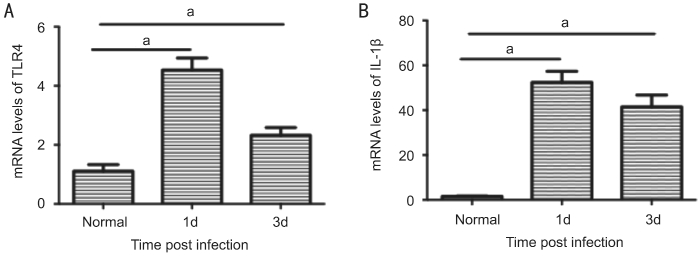
Enhanced Toll-like Receptor 4 and Interleukin-1β Production During Aspergillus fumigatus Infection We examined the mRNA expression of TLR4 and IL-1β in normal and A.fumigatus-infected corneal tissue using real-time RT-PCR. The mRNA levels were significantly increased in A. fumigatusinfected corneal tissue. Compared to those in the normal control, the relative TLR4 (P<0.001; Figure 1A) and IL-1β(P<0.001; Figure 1B), mRNA levels were significantly higher in C57BL/6 mice corneal tissues on days 1 and 3 after infection,and both mRNA levels peaked on day 1 after infection.
Disease Responses in C57BL/6 Mouse Corneal Tissues After Rapamycin Treatment To elucidate the role of mTOR in A. fumigatus-induced inflammatory responses, rapamycin was used to inhibit the activity of mTOR in C57BL/6 mice corneal tissues. The effect of rapamycin on the outcome of fungal keratitis was assessed by photographs taken using a slit-lamp (Figure 2A-2C) and clinical score analysis (Figure 2D). The results of the photograph analysis showed that the severity of the disease decreased after rapamycin treatment on day 1 p.i. (Figure 2C) compared with that on treatment with the vehicle (Figure 2B). A significant difference was also observed on the analysis of clinical scores (P<0.05; Figure 2D). Further, Western blot con fi rmed the effect of rapamycin on mTOR expression in C57BL/6 mice corneal tissues on day 1 p.i. mRNA and protein levels of mTOR were assessed using real-time RT-PC R and Western blot, respectively (Figure 2A,2B). There was no significant difference in mTOR expression between the vehicle and rapamycin-treated groups (Figure 2E-2G); however, Western blotting showed that rapamycin treatment downregulated p-mTOR protein levels in infected and uninfected corneal tissues compared to the vehicle group(Figure 2F, 2H).
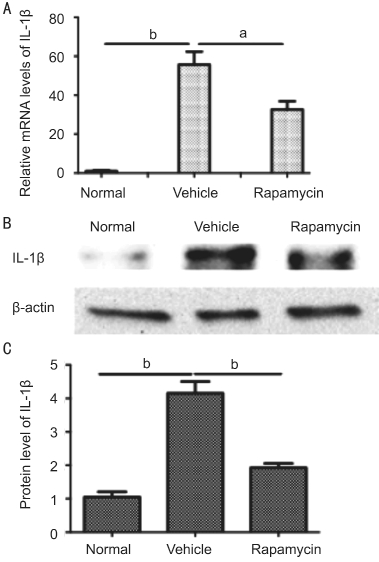
Effect of Rapamycin Treatment on Expression of Interleukin-1β The relative mRNA levels of the proinflammatory cytokine, IL-1β, were measured in the infected corneal tissue of C57BL/6 mice on day 1 p.i. by RT-PCR.Rapamycin treatment significantly suppressed the expression of IL-1β compared to the vehicle (P<0.01; Figure 3A). The protein levels of IL-1β stimulated by A. fumigatus were also significantly reduced following rapamycin treatment (P<0.001;Figure 3B, 3C).
Inhibitory Effect of Rapamycin on TLR4 Upregulated by Aspergillus Fumigatus To investigate whether the reduced expression of IL-1β after inhibition of mTOR expression is associated with TLR4, relative mRNA levels (Figure 4A) or protein levels (Figure 4B, 4C) of TLR4 were assessed by RTPCR an d Western blot, respectively. After rapamycin treatment,there was a significant decrease in the mRNA levels on day 1 after infection (P<0.01; Figure 4A) compared with the vehicle group. Protein expression of TLR4 was also significantly decreased on day 1 after infection compared to that in the vehicle group, as observed by Western blot (P<0.001; Figure 4B, 4C).
DISCUSSION
Fungal keratitis is a challenging ophthalmologic condition that requires aggressive treatment to prevent untoward outcomes[41].Infection with Aspergillus species is one of the major causes of funngal keratitis worldwide, resulting in visual impairment and even blindness. Research on the exact pathogenesis of Aspergillus-induced corneal infections is thus of great significance.
Considering the high molecular weight of rapamycin and the barrier structure of the eye, we selected subconjunctival injection in mice before infection in order to ensure the drug could effectively reach the cornea. Changes in the expression of mTOR and p-mTOR after rapamycin intervention were detected at the beginning, and they were considered to be indicative of the inhibition of the mTOR signaling pathway;these observations also verified the model of drug intervention in mice. Studies have reported that the mTOR inhibitor,rapamycin, only inhibited expression of p-mTOR, but not of unphosphorylated mTOR[25,42-43]. Our data also showed that the mRNA and protein levels of mTOR were not significantly different between the vehicle and rapamycin-treated groups;however, rapamycin treatment downregulated p-mTOR expression compared to the vehicle group.
A retinal inflammation study indicated that the expression of inflammatory molecules was suppressed by rapamycin in the retina during inflammation[39]. Similarly, a study of LPS-induced acute lung injury suggested that knockdown of mTOR reduces LPS-induced inflammatory responses in human bronchial epithelial (HBE) cells[25]. Another study showed that the decreased expression levels of mTOR reduced the concentrations of IL-1β and IL-6 in a rat model of spinal cord injury[44]. Other studies have shown that rapamycin treatment suppressed the expression of immunostimulatory molecules in in vitro-generated human monocyte-derived dendritic cells during LPS stimulation and inhibited the expression of IL-12p40, TNF,and IL-6[10,45]. IL-1β is an important pro-inflammatory cytokine in the inflammatory response against fungal infections[9,31-32].In this process, upon β-glucan stimulation, TLR4 triggers NF-κB and other signaling pathways. TLR4-dependent activation of the signaling network triggers IL-1β induction. In our study,treatment with rapamycin reduced the expression of p-mTOR,A. fumigatus-induced TLR4, and IL-1β production after infection. Our in vivo experiments suggested that p-mTOR mediated the induction of IL-1β, likely through the mTOR/TLR4 signaling pathway. These results indicate that mTOR participates in the TLR4-induced IL-1β induction in mouse A. fumigatus-induced keratitis, supplementing a regulatory pathway for IL-1β induction triggered by TLR4. However, a study on Pseudomonas aeruginosa-induced keratitis showed IL-10 mRNA expression was significantly decreased after rapamycin treatment and that rapamycin treatment leads to the development of a pro-inflammatory environment in BALB/c mice[38]. This conclusion appears slightly different from our findings. We hypothesized that this discrepancy could be attributed to the different mouse models, different methods of
drug interventions, different drug concentrations, and different disease agents (bacteria or fungi).
Therefore, our study showed that the expression of TLR4 and pro-inflammatory cytokine IL-1β, was significantly upregulated during Aspergillus infection of the cornea in mice,while rapamycin suppressed this elevation in expression.These data suggest a potential role of mTOR in mediating inflammatory responses in A. fumigatus-induced keratitis in mice and prove that rapamycin has an anti-inflammatory effect, which may be mediated via the mTOR/TLR4 pathway in A. fumigatus-induced keratitis in mice. Furthermore, our results propose the potential use of rapamycin treatment as a therapy for suppressing the expression of p-mTOR and related inflammatory molecules, thus preventing subsequent visual dysfunction during fungal keratitis. There is clearly more than one mechanism by which rapamycin may regulate innate immunity, and further studies must be conducted to explore these mechanisms.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81500695).
Conflicts of Interest: Xu R, None; Lin J, None; Zhao GQ,None; Li C, None; Che CY, None; Xu Q, None; Liu M, None.
REFERENCES
1 Jiang N, Zhao G, Lin J, Hu L, Che C, Li C, Wang Q, Xu Q, Peng X.Indoleamine 2,3-Dioxygenase is involved in the inflammation response of corneal epithelial cells to Aspergillus fumigatus infections. PLoS One 2015;10(9):e0137423.
2 Peng XD, Zhao GQ, Lin J, Jiang N, Xu Q, Zhu CC, Qu JQ, Cong L,Li H. Fungus induces the release of IL-8 in human corneal epithelial cells, via Dectin-1-mediated protein kinase C pathways. Int J Ophthalmol 2015;8(3):441 -447.
3 Li C, Zhao G, Che C, Lin J, Li N, Hu L, Jiang N, Liu Y. The role of LOX-1 in innate immunity to Aspergillus fumigatus in corneal epithelial cells. Invest Ophthalmol Vis Sci 2015;56(6):3593-3603.
4 Wang N, Zhao GQ, Gao A, Che CY, Qu XL, Liu Y, Guo YL. Association of TLR2 and TLR4 gene single nucleotide polymorphisms with fungal keratitis in Chinese Han population. Curr Eye Res 2014;39(1):47-52.
5 Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116(11):3015-3025.
6 Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1(2):135-145.
7 Lee SM Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog 2010;6:e1000976.
8 Jie Z, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun 2009;15(3):155-168.
9 Gao X, Zhao G, Li C, Lin J, Jiang N, Wang Q, Hu L, Xu Q, Peng X, He K,Zhu G. LOX-1 and TLR4 affect each other and regulate the generation of ROS in A. fumigatus keratitis. Int Immunopharmacol 2016;40:392-399.
10 Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol 2015;15(10):599-614.
11 Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149(2):274-293.
12 Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 2014;15(3):155-162.
13 Yang J, Zhao X, Patel A, Potru R, Azizi-Ghannad S, Dolinger M, Cao J, Bartholomew C, Mazurklewlcz J, Conti D, Jones D, Huang Y, Zhu XC.Rapamycin inhibition of mTOR reduces levels of the Na+/H+ exchanger 3 in intestines of mice and humans, leading to diarrhea. Gastroenterology 2015;149(1):151-162.
14 Raïch-Regué D, Rosborough BR, Watson AR, Mcgeachy MJ,Turnquist HR, Thomson AW. mTORC2 Deficiency in myeloid dendritic cells enhances their allogeneic Th1 and Th17 stimulatory ability after TLR4 ligation in vitro and in vivo. J Immunol 2015;194(10):4767-4776.
15 Sinclair C, Bommakanti G, Gardinassi L, Loebbermann J, Johnson MJ, Hakimpour P, Hagan T, Benitez L, Todor A, Machiah D, Oriss T,Ray A, Bosinger S, Ravindran R, Li S, Pulendran B. mTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic in fl ammation. Science 2017;357(6355):1014-1021.
16 Lin HY, Chang KT, Hung CC, Kuo CH, Hwang SJ, Chen HC, Hung CH, Lin SF. Effects of the mTOR inhibitor rapamycin on monocytesecreted chemokines. BMC Immunol 2014;15:37.
17 do Nascimento de Freitas D, Gassen RB, Fazolo T, Souza APD.Rapamycin increases RSV RNA levels and survival of RSV-infected dendritic cell depending on T cell contact. Toxicol In Vitro 2016;36:114-119.
18 Mineharu Y, Kamran N, Lowenstein PR, Castro MG. Blockade of mTOR signaling via rapamycin combined with immunotherapy augments antiglioma cytotoxic and memory T-cell functions. Mol Cancer Ther 2014;13(12):3024-3036.
19 Pinelli DF, Wakeman BS, Wagener ME, Speck SH, Ford ML.Rapamycin ameliorates the CTLA4-Ig-mediated defect in CD8(+) T cell immunity during gammaherpesvirus infection. Am J Transplant 2015;15(10):2576-2587.
20 Schwaiger T, van den Brandt C, Fitzner B, Zaatreh S, Kraatz F,Dummer A, Nizze H, Evert M, Bröker BM, Brunner-Weinzierl MC,Wartmann T, Salem T, Lerch MM, Jaster R, Mayerle J. Autoimmune pancreatitis in MRL/Mp mice is a T cell-mediated disease responsive to cyclosporine A and rapamycin treatment. Gut 2014;63(3):494-505.
21 Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M,Stuhlmeier KM, Kolbe T, Stulnig TM, Hörl WH, Hengstschläger M,Müller M, Säemann MD. The TSC-mTOR signaling pathway regulates the innate in fl ammatory response. Immunity 2008;29(4):565-577.
22 Lorne E, Zhao X, Zmijewski JW, Liu G, Park YJ, Tsuruta Y, Abraham E. Participation of mammalian target of rapamycin complex 1 in Toll-like receptor 2- and 4-induced neutrophil activation and acute lung injury. Am J Respir Cell Mol Biol 2009;41(2):237-245.
23 Janes MR, Fruman DA. Immune regulation by rapamycin: moving beyond T cells. Sci Signal 2009;2(67):pe25.
24 Laberge RM, Sun Y, Orjalo AV, et al. MTOR regulates the protumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015;17(8):1049-1061.
25 Hu Y, Lou J, Mao YY, et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 2016;12(12):2286-2299.26 Zhang C, Wang S, Li J, Zhang W, Zheng L, Yang C, Zhu T, Rong R. The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury.Cell Death Dis 2017;8(3):e2695.
27 Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A,Thomson AW. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo.Blood 2003;101(11):4457-4463.
28 Schmitz F, Heit A, Dreher S, Eisenächer K, Mages J, Haas T, Krug A,Janssen KP, Kirschning CJ, Wagner H. Mammalian target of rapamycin(mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol 2008;38(11):2981-2992.
29 Gabrion A, Hmitou I, Moshous D, Neven B, Lefèvreutile A,Diana JS, Suarez F, Picard C, Blanche S, Fischer A, Cavazzana M,Touzot F. Mammalian target of rapamycin inhibition counterbalances the inflammatory status of immune cells in patients with chronic granulomatous disease. J Allergy Clin Immunol 2017;139(5):1641-1649.
30 Chung EJ, Sowers A, Thetford A, McKay-Corkum G, Chung SI,Mitchell JB, Citrin DE. Mammalian target of rapamycin inhibition with rapamycin mitigates radiation-induced pulmonary fibrosis in a murine model. Int J Radiat Oncol Biol Phys 2016;96(4):857-866.
31 Underhill D, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 2015;43(5):845-858.
32 Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, Van dVM,Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 in fl ammasome. Nat Immunol 2012;13(3):246-254.
33 Lee HS, Hattori T, Park EY, Stevenson W, Chauhan SK, Dana R.Expression of toll-like receptor 4 contributes to corneal in fl ammation in experimental dry eye disease. Invest Ophthalmol Vis Sci 2012;53(9):5632-5640.
34 Karthikeyan RS, Leal SM Jr, Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, Lalitha P. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium.J Infect Dis 2011;204(6):942-950.
35 Sun Q, Liu Q, Zheng Y, Cao X. Rapamycin suppresses TLR4-triggered IL-6 and PGE(2) production of colon cancer cells by inhibiting TLR4 expression and NF-kappaB activation. Mol Immunol 2008;45(10):2929-2936.36 Ren G, Hu J, Wang R, Han W, Zhao M, Zhou G, Zhang C, Zhang Z.Rapamycin inhibits Toll-like receptor 4-induced pro-oncogenic function in head and neck squamous cell carcinoma. Oncol Rep 2014;31(6):2804-2810.
37 Yu R, Bo H, Villani V, Spencer PJ, Fu P. The inhibitory effect of rapamycin on Toll like receptor 4 and interleukin 17 in the early stage of rat diabetic nephropathy. Kidney Blood Press Res 2016;41(1):55-69.
38 Foldenauer ME, McClellan SA, Berger EA, Hazlett LD. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J Immunol 2013;190(11):5649-5658.
39 Okamoto T, Ozawa Y, Kamoshita M, Osada H, Toda E, Kurihara T, Nagai N, Umezawa K, Tsubota K. The neuroprotective effect of rapamycin as a modulator of the mTOR-NF-κB axis during retinal in fl ammation. PLoS One 2016;11(1):e0146517.
40 Zhou Z, Barrett R, McClellan SA, Zhang Y, Szliter EA, van Rooijen N, Hazlett LD. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci 2008;49(10):4458-4467.
41 Jeng BH. Challenges in the Management of fungal keratitis. JAMA Opthalmol 2017;135(6):525-526.
42 Shiu SW, Wong Y, Tan KC. Effect of advanced glycation end products on lectin-like oxidized low density lipoprotein receptor-1 expression in endothelial cells. J Atheroscler Thromb 2012;19(12):1083-1092.
43 Wang H, Duan L, Zou Z, Li H, Yuan S, Chen X, Zhang Y, Li X, Sun H, Zha H, Zhang Y, Zhou L. Activation of the PI3K/Akt/mTOR/p70S6K pathway is involved in S100A4-induced viability and migration in colorectal cancer cells. Int J Med Sci 2014;11(8):841-849.
44 Yang Y, Guo C, Liao B, Cao J, Liang C, He X. BAMBI inhibits in fl ammation through the activation of autophagy in experimental spinal cord injury. Int J Mol Med 2017;39(2):423-429.
45 Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M,Zlabinger GJ, Pulendran B, Hörl WH, Säemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol 2010;185(7):3919-3931.
Co-first authors: Rui Xu and Jing Lin
Correspondence to: Gui-Qiu Zhao. Department of Ophthalmology, the Affiliated Hospital of Qingdao University,Qingdao 266003, Shandong Province, China. Zhaoguiqiu_good@126.com
Rece ived:2017-10-23
Accepted:2018-03-19
DOl:10.18240/ijo.2018.05.02
Citation: Xu R, Lin J, Zhao GQ, Li C, Che CY, Xu Q, Liu M. Production of interleukin-1β related to mammalian target of rapamycin/Toll-like receptor 4 signaling pathway during Aspergillus fumigatus infection of the mouse cornea. Int J Ophthalmol 2018;11(5):712-718