INTRODUCTION
Alterations in the choroid vascular structure and blood flow are associated with myopic refractive errors[1-6]. In monkeys, contact lens-induced myopia causes the choroid to become thinner, and this thinning is reversed when the lens is removed[7]. The same result was found in the study of chicks[8].Summers hypothesized that the choroid may be a source of growth factors that influence scleral development[9].
Though choroid was be supposed to play some role in myopia progression, the detection technology was limited to be used in human being until optical coherence tomography (OCT) was invented and applied to ophthalmology. Due to the attenuation,inchoate OCT could not detect the boundaries of choroid.Recently, advances such as enhanced depth imaging optical coherence tomography (EDI-OCT) enabled measurement of choroid thickness in both children and adults[3,5,10]. These studies consistently showed that the choroid was thinner in myopic subjects, most of whom wore spectacles for myopia correction, compared to emmetropes[1-3,5]. The thinner choroid observed in the myopic children supports a potential role for the choroid in the development and progression of myopia[1].
Myopia is one of the most common ocular disorders, and in China, it affects about 40%-96.9% of the population[11-12]. Many methods have been used to slow the progression of myopia.Among these methods, orthokeratology (Ortho-K) is widely used in China[13]. Ortho-K is a technology for temporarily correcting low to moderate degrees of myopia using specially designed reverse-geometry contact lenses[13-15]. The effects of this modality on the correction of myopic refractive error have been well documented in previous studies[14-16], and the ability to reduce the progression of myopia has been documented for many years[17-21]. Most children who wearing Ortho-K lens could gain good visual acuity during daytime[13,15,22]. Some studies have shown that the increase in axial length (AL) is lower with Ortho-K lens wear than with spectacles. Even when Ortho-K lens was compared to atropine, the AL increased in the group of Ortho-K lens was shorter than atropine[21]. While the mechanism by which the axial lengthening is slowed was not clear[20].
For choroid might play some roles in myopia, maybe Ortho-K lens controlling myopia was due to the change of choroid.Choroid image could be caught by OCT and the choroid in horizontal meridian was easy acquired than that in vertical meridian for the eyelids were small in part of subjects.Therefore, the aim of this study was to use EDI-OCT to evaluate choroidal thickness in the horizontal meridian and analyze the correlation of choroid thickness change with changes in the AL, refractive error, and corneal curvatures.
SUBJECTS AND METHODS
Subjects This cross-sectional study was approved by the Office of Research Ethics, Wenzhou Medical University and registered in the Chinese Clinical Trial Registry (registry number: ChiCTR-RON-15007389). Informed consent was obtained from each subject and their parents. All subjects and their parents were treated in accordance with the tenets of the Declaration of Helsinki.
A total of 30 subjects (30 eyes) without history of contact lens wear or any current ocular or systemic disease were enrolled. There were 16 females and 14 males, and the mean age for all subjects was 11.3±1.7y (range, 9 to 14y). Each subject had myopia of -1 to -6 diopters (D) and astigmatism of no more than -1.00 D. The refractive error was measured not under cycloplegia. After auto refraction, subjective refraction was carried out. All of the subjects underwent examination by an experienced optometrist (Jin WQ) with a slit-lamp biomicroscope, and the refractive error for each was determined. Subjects were enrolled in the study only if the prescribed Ortho-K lenses fitted optimally, characterized by good on-eye centration as determined by the fluorescein staining pattern and topography.
All subjects had best corrected visual acuity in both eyes of 0.0 logMAR or better, and none had evidence or history of significant ocular or systemic disease. All subjects underwent an ophthalmic screening examination including visual acuity, non-cycloplegic manifest subjective refraction, and ocular health status. All measurements were carried out between 2:00 and 5:00 p.m. to limit the potential influence of diurnal variations of choroidal thickness[4]. Non-cycloplegic refractive error was taken in this study. For this was the most natural condition of the subjects in the study and the accommodation was controlled and reduced impact by the experienced optometrists. Central corneal thickness (CCT),flat keratometry (K1), steep keratometry (K2), lens thickness(LT), anterior chamber depth (ACD), AL, and white-towhite (W-W) distance were measured with a Lenstar LS 900 Biometer (Haag-Streit AG, Koeniz, Switzerland), and the data were recorded automatically. Each subject wore Euclid Ortho-K lenses (Herndon, VA, USA) in both eyes. The lenses were composed of Equalens II with a gas permeability of 120×10-11 (cm2·mLO2)/(s·mL·mm Hg). Trial lenses were initially fitted and the centration and fluorescein staining pattern were assessed by one of the authors (Jin WQ). Ortho-K lenses for both eyes were dispensed to be worn overnight and removed soon after eye opening for three months.
The group control was carried out through the following methods. All of the measurements were operated by the same operator. The eye tracing function of the instrument was used to make sure the same location of choroid image. Each parameter was measured three times and the average was used to statistical analysis.
Choroid Image and Thickness Profile A commercial spectral domain OCT instrument (Cirrus-HD OCT, Carl Zeiss Meditec, Inc., Dublin, CA, USA) was used to assess the choroidal thickness profiles obtained from cross-sectional images. The ability of the Cirrus-HD instrument to measure the thickness of the choroid with good repeatability has been documented[23]. The intensity of each image was automatically scored from 1-10, which was used to judge the image quality.Choroid thickness images which at least 6 out of 10 in intensity were thought achieving the measure quality. The detailed configuration of the OCT instrument was reported in a previous study[24]. In brief, the images were captured in the horizontal meridian. The subjects were asked to look straight ahead, and the OCT beam was aligned and set across the fovea.The HD-5 line mode was chosen. The image scan field was set for 6-mm width and 2-mm depth (256×256 pixels). The light source was centered at a wavelength of 840 nm, and the axial resolution in tissue was 5 μm. To facilitate the measurement of choroid thickness, the interface between the choroid and the sclera was visualized by image enhancement software. In this study, the images were captured without pupil dilation because dilation had no effect on choroid thickness measurements[25].To ensure image quality, only images with intensity ratings of≥6 were chosen for analysis[26]. Possible measurement errors were minimized by the eye tracing ability of OCT, and the same operator carried out all of the choroid imaging. The good repeatability of the Cirrus-OCT had been shown in a previous study[26].
The choroid thickness for an approximately 6-mm chord in the sub-fovea was imaged, but only the central 3.5-mm region was analyzed due to loss of optical information in the peripheral region. The boundary of the choroid were detected by using an automatic segmentation method based on graph-search theory as described in detail in prior publication[27]. A manual approach was incorporated into the algorithm to correct for any minor segmentation errors.
Analysis was carried out using custom software on images in which the software clearly detected the choroid border in the horizontal (Figure 1). The choroid around the fovea, equivalent to a 3.5-mm chord distance was selected for data analysis.
Table 1 Biometric parameters and changes during three months of overnight Ortho-K lens wear

CCT: Central corneal thickness; K1: Flat keratometry; K2: Steep keratometry; LT: Lens thickness; ACD: Anterior chamber depth; AL: Axial length; W-W: White-to-white corneal distance. P-values are comparisons between baseline and after three months of Ortho-K lens wear.
Table 2 Horizontal meridian choroid thickness before and after Ortho-K lens wear μm
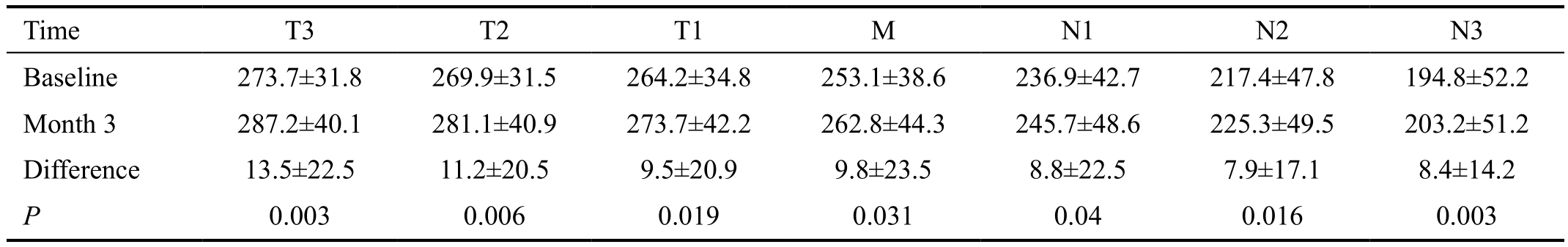
T: Temporal; M: Macula; N: Nasal. P-values are comparisons between baseline and after three months of Ortho-K lens wear.
The analysis region along the horizontal meridian was divided into seven 500-µm wide zones from the temporal (T) to the nasal (N) sides (Figure 1): T3, T2, T1, M, N1, N2, and N3.In the OCT image, fovea, the center of macula was highly reflective, similar to that seen with a funduscope. To ensure the reproducibility of the OCT images, the operator captured the images when the macular reflex was present.
The images of each subjects before and after 3mo were captured by the same operator who named Xiao-Li Li, a college in our hospital clinical research centre. The image processing were carried out by two trained students respectively. The average of the two result was used to analyse.
Data Analysis SPSS software (Version 18.0 for Windows,SPSS Inc., Chicago, IL, USA) was used for descriptive statistics and data analysis. Quantitative variables such as age, refractive error, and choroidal thickness were expressed as means±standard deviations. The choroidal thickness of only the right eye was analyzed. The paired t-test was used to compare the choroidal thicknesses at baseline and after three months of overnight Ortho-K lens wear. Statistical significance was accepted when P<0.05. Tukey’s test was used to compare the choroid thickness difference in the zones of horizontal meridian. Pearson’s correlation coefficient was used to analyse the correlation of the choroid thickness increase and AL change.
RESULTS
The average myopia for the enrolled subjects was -2.9±1.1 D(range, -1.00 to -6.00 D). After Ortho-K lens wear, the all subjects’ visual acuity without correction were better than 0 logMAR. At baseline, the CCT was 552.60±32.10 µm,and after three months of Ortho-K lens wear, it decreased 6.30±7.02 µm (P<0.01; Table 1). Similarly, the baseline K1 and K2 were 41.44±1.86 D and 42.42±2.08 D respectively,and each decreased 1.29±1.73 D and 1.08±1.73 (P<0.01 and P=0.002 respectively; Table 1). The values for ACD, AL, pupil diameter, and W-W did not change significantly over the period of the study. The values for LT show a tiny but sighificantly change after OK lens wear.
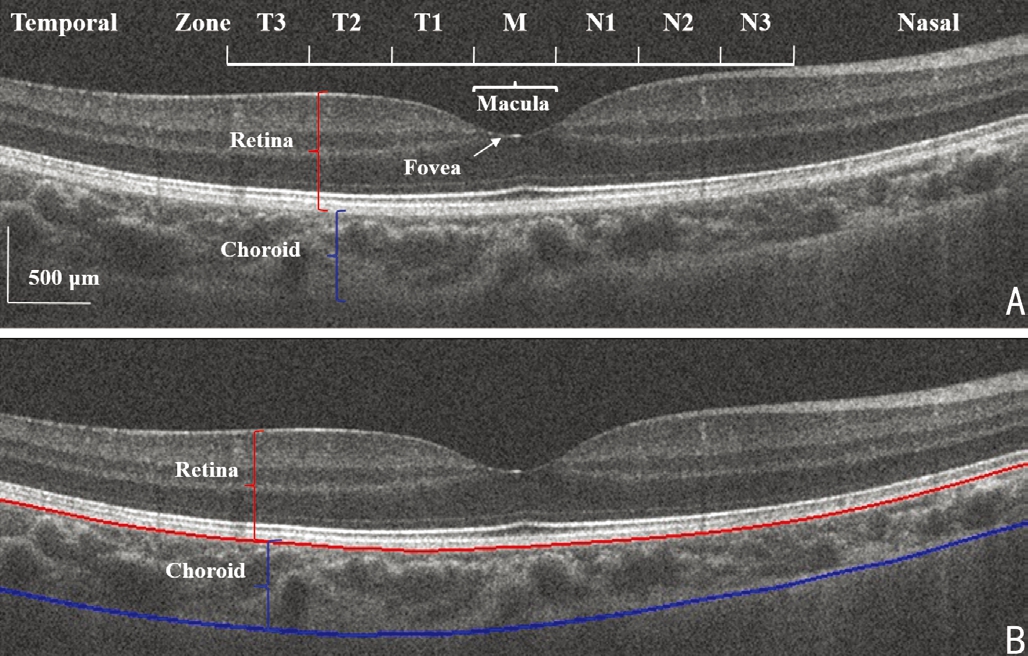
Figure 1 Choroid image captured by OCT in the horizontal meridian A: Original OCT image divided into temporal (T) and nasal (N) zones with the fovea in the macula (M) center; B: Choroid boundary as detected by the software. The greater thickness of the temporal choroid compared to the nasal choroid is evident. Interval between each zone is 500 µm; Bar, 500 µm.
Choroid Thickness Prior to wearing the Ortho-K lenses,the baseline thickness of the choroid in the horizontal meridian was greatest at T3 (273.7±31.8) μm, and it thinned progressively through the fovea and the nasal zones (Table 2).The thinnest choroid was at N3 (194.8±52.2) μm, which was significantly thinner than T3 (P<0.05, Tukey’s test). After three months of Ortho-K lens wear, the choroid thickness in each of the horizontal meridional zones was significantly thicker than the baseline values (Table 2). The greatest increase in thickness was in T3 and the smallest increase was in N2. The increased choroid thickness were shown in Figure 2.
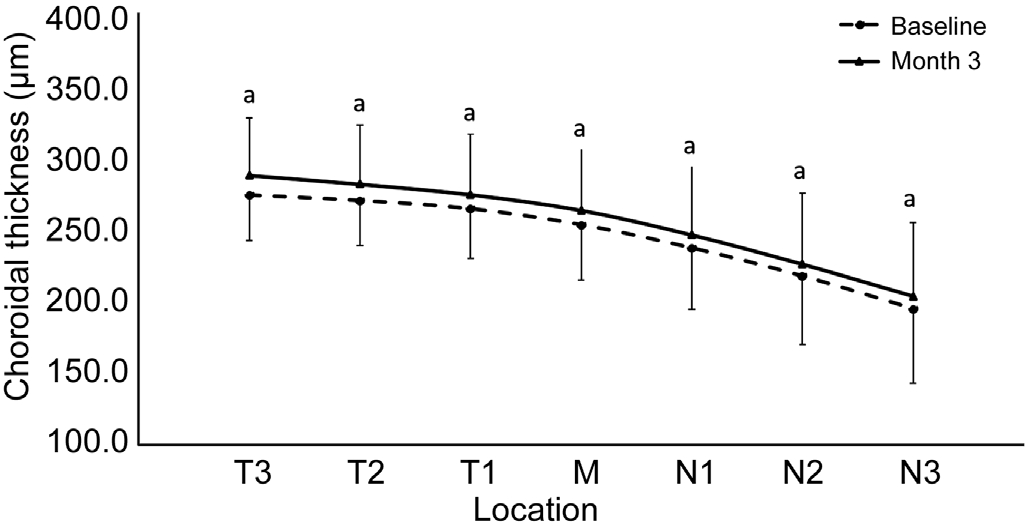
Figure 2 Comparing the choroid thicknesses before and after Ortho-K lens wear Both of before and after Ortho-K lens wear,thicker in temporal zones and thinner in nasal zones. The choroid thickness increased in each zones at horizontal meridian. aP<0.05. N:Nasal; T: Temporal.
Correlation of Choroid Thickness with Ocular Biometry After three months of Ortho-K lens wear, there was a significant negative correlation between the changes in the AL and the changes in choroid thickness for each of the zones in the horizontal meridian (r: -0.3 to -0.4, P<0.05; Figure 3)except for N3 (r: -0.22, P>0.05).
DISCUSSION
As in previous studies[13,15,22], the Ortho-K lens changed the corneal topography and increased uncorrected visual acuity. In this study, the K1 and K2 became more flat after lens wear, and the visual acuity increased significantly. The most interestingly in this study, the choroid thickness increased in the horizontal meridian. To our knowledge, this was the first study to measure changes in the thickness of the choroid in the horizontal meridian after Ortho-K in children with progressive myopia which sample size larger than 30. Before wearing the Ortho-K lenses, the choroid in most temporal zones of the horizontal meridian was thicker than nasal zones, the thickest zone was temporal 3 zone, and it was progressively thinning from the temporal to the nasal side. This finding was consistent with other studies of choroid thickness[1-3,5]. However there were some different results about the choroid thickness distribution.In the study of Margolis and Spaide[28], investigate adults with refractive errors, the thickest choroid was present in the subfovea. Cross-sectional studies of myopic adults have reported that the temporal choroid is relatively thick compared to the nasal choroid, and that the choroid in the superior zones is thicker than in the inferior zones[29-30]. Read et al[1] observed significant variations in the topographic distribution of choroid thickness. The choroid in the nasal region was relatively thin,while the superior-temporal regions were slightly thicker.Differences in choroid thickness were not strictly associated with horizontal meridian or the quadrants defined by them.They suggested that there are regional differences in the choroid architecture associated with myopia and that the central choroid plays a more important role in the progression of myopia than does the peripheral regions. These regional differences could be explained by the fact that the macula has a larger amount of nonvascular smooth muscle cells than do the peripheral regions[31].
The choroid also contains non-vascular smooth muscle cells,especially behind the macula[31-32]. Contraction of these cells may make the choroid thin, thereby opposing the thickening caused by expansion of the choroid capillary vessel lacunae.There are intrinsic choroid neurons, also mostly behind the central retina, that control these muscle cells and may modulate choroid blood flow as well[7,31-32]. The choroid, which is the major supplier of blood for the outer retina, also contains secretory cells that are probably involved in the modulation of vascularization and in the growth of the sclera[9,32]. Finally,dramatic changes in choroid thickness can move the retina forward or backward, changing the photoreceptor plane of focus[31-33]. This function is demonstrated by the thinning of the choroid that occurs when the focal plane is moved back by the wearing of myopia Ortho-K lenses, and, conversely, by the thickening that occurs when positive lenses are worn[32,34-35].
Choroid thickness changes also are correlated with changes in the growth of the sclera, and hence of the eye[1-2,9]. Transient increases in choroid thickness are followed by prolonged decreases in synthesis of extracellular matrix molecules and a slowing of ocular elongation[32]. Attempts to decouple the choroid and scleral changes have largely failed, and it seems that the thickening of the choroid may be mechanistically linked to the scleral synthesis of macromolecules[28,32,36]. Thus the choroid may play an important role in the homeostatic control of eye growth, and consequently, in the etiology of myopia[32]. In myopic children, capillary vessel thinning was closely related axial elongation[37].
After three months of nightly Ortho-K lens wear, choroid thickness increased for all of the seven zones in the horizontal meridian. In contrast, Gardner et al[35] found no significant thickening of the choroid after 9mo of Ortho-K lens wear,although the macular thickened by 6 µm. In our study, the thickness of the choroid in the macula increased by 9.8 µm.We used custom software to acquire the choroid thickness profile in a 3.5-mm field, and the thickness was determined in 0.5-mm wide zones. This method decreased the inherent error associated with single point measurements. The sample size in our study was 30 with a mean age of 11.3±1.7y, while the subjects in Gardner et al’s[35] study was only 9 and with average age of 13.6±1.3y. The younger age of our subjects could mean that the myopia was developing faster. Furthermore, the larger number of subjects in our study may have improved the likelihood that we would detect the significant changes in choroid thickness. Because of eye movements, Gardner et al[35] may have included measurements of peripheral choroid thickness that could have influenced the estimates of actual thickness. In contrast, in our protocol the image field was 6 mm and captured the entire macula in a single shot as the eye looked straight ahead. Thus, there was no discontinuity or overlap of the images in different zones along the horizontal or meridian.
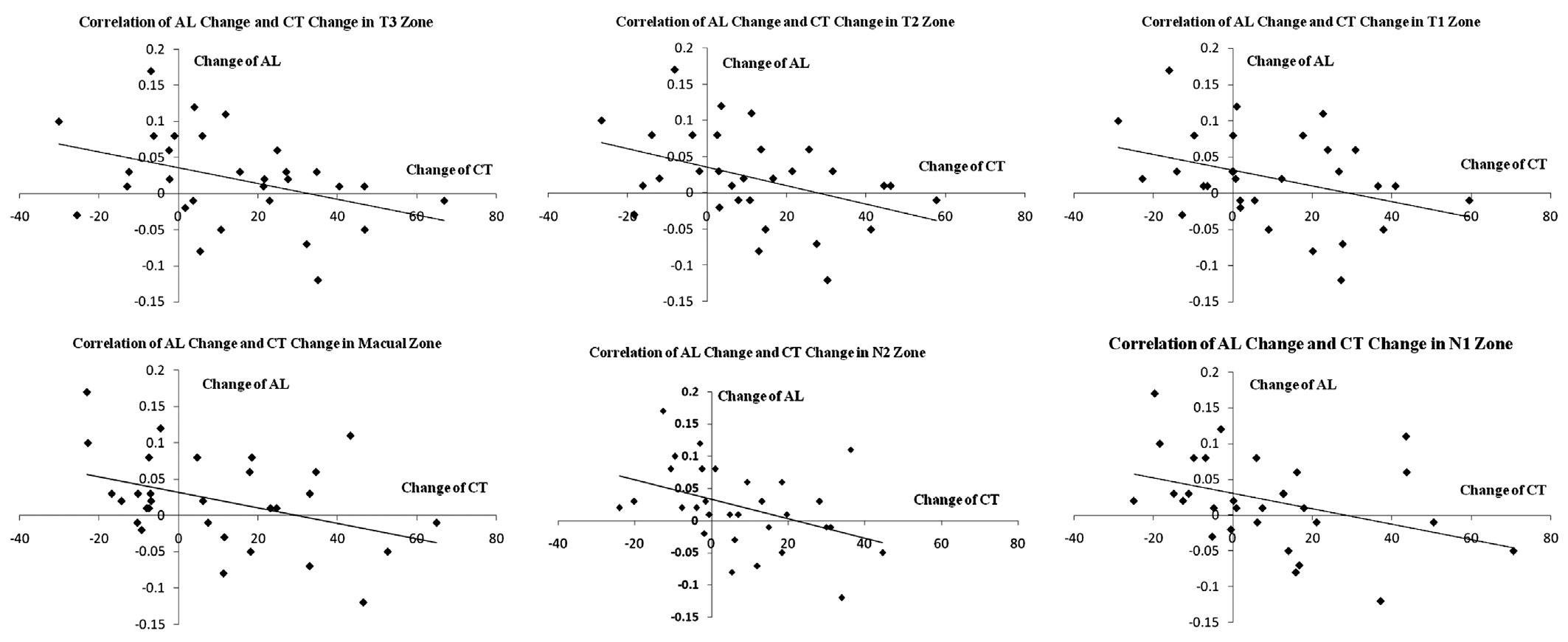
Figure 3 Correlation of choroid thickness with axial length in the horizontal meridian For each of the zones in the horizontal meridian except N3, there was a significant negative correlation between the changes in the AL and the changes in choroid thickness. For all zones, the r-values were as follows: T3, r=-0.38; T2, r=-0.41; T1, r=-0.36; Macula, r=-0.40; N1, r=-0.38; N2, r=-0.41; N3, r=-0.22. Except for N3, all P-values were <0.05. AL: Axial length; CT: Choroid thickness; N: Nasal; T: Temporal.
Measurement of AL is the gold standard for evaluating the progression of myopia, and many cross-sectional studies reported that the AL is greater in myopic eyes than in normal eyes[1-3,5]. Further, several studies have reported that Ortho-K lens wear suppresses increase of AL in myopic children[34-36].In contrast, Gardner et al[33] found that Ortho-K induced no consistent, significant changes in either ocular length or choroid thickness associated with the progression of myopia[33].However our results clearly showed that in the horizontal meridian, the increase in choroid thickness for all zones except N3 was negatively correlated with the changes in AL.
The vessels of the choroid indirectly supply the outer retina and directly supply the macular arch ring. The retinal changes and choroid degeneration in myopic eyes may be related to the dysfunction of the blood flow. During myopia progression,choroid atrophy is most evident on the temporal side, possibly due to the stretching of the temporal choroid and sclera, which can cause thinning of the choroid. This suggests that during myopia progression, the temporal choroid thickness is more sensitive to the optical stimulus changes than the nasal choroid.We found that after three months of treatment, the increase in choroid thickness was greatest in the temporal zones, and this suggests that this region is more responsive to Ortho-K lens wear[34].
In summary, after three months of overnight Ortho-K lens wear, there was an increase in the choroid thickness profile along the horizontal meridian. While changes in the AL were not significant during the study period, they were negatively correlated with the increased thickness of the choroid. These findings are consistent with a potential role of the choroid in the development of human refractive error. For the period of this study is only three months and the sample is 30,longitudinal and more samples size studies during childhood are required to further our understanding of the relationship between the thickness of the choroid and the development and progression of refractive errors.
ACKNOWLEDGEMENTS
Authors’ contributions Design of the study: Lian Y, Jin WQ;Performed the experiments: Lian Y, Data analysis: Huang SH,Jiang J, Mao XJ, Shen MX; Wrote the paper: Lian Y, Jin WQ.Foundations: Supported by National Health and Family Planning Commission of the People’s Republic of China(No.201302015); Zhejiang Provincial Natural Science Foundation of China (No.LY14H120007); Wenzhou Commonweal Technology Project (No.Y20150253); Eye Hospital of Wenzhou Medical University Innovation Grant(No.YNCX201402).
Conflicts of Interest: Jin WQ, None; Huang SH, None;Jiang J, None; Mao XJ, None; Shen MX, None; Lian Y,None.
REFERENCES
1 Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 2013;54(12):7578-7586.
2 Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in childhood. Invest Ophthalmol Vis Sci 2013;54(5):3586-3593.
3 Ding X, Li J, Zeng J, Ma W, Liu R, Li T, Yu S, Tang S. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci 2011;52(13):9555-9560.
4 Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length,choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci 2011;52(8):5121-5129.
5 Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci 2010;51(4):2173-2176.
6 Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography.Am J Ophthalmol 2010;150(3):325-329.e1.
7 Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci 2000;41(6):1249-1258.
8 Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res 1995;35(9):1175-1194.
9 Summers JA. The choroid as a sclera growth regulator. Exp Eye Res 2013;114(9):120-127.
10 Fujiwara A, Shiragami C, Shirakata Y, Manabe S, Izumibata S,Shiraga F. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes.Jpn J Ophthalmol 2012;56(3):230-235.
11 Lin LL, Shih YF, Tsai CB, Chen CJ, Lee LA, Hung PT, Hou PK.Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci 1999;76(5):275-281.
12 Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050.Ophthalmology 2016;123(5):1036-1042.
13 Cho P, Cheung SW, Mountford J, White P. Good clinical practice in orthokeratology. Cont Lens Anterior Eye 2008;31(1):17-28.
14 Nichols JJ, Marsich MM, Nguyen M, Barr JT, Bullimore MA.Overnight orthokeratology. Optom Vis Sci 2000;77(5):252-259.
15 Swarbrick HA, Wong G, O’Leary DJ. Corneal response to orthokeratology. Optom Vis Sci 1998;75(11):791-799.
16 Walline JJ, Rah MJ, Jones LA. The Children’s Overnight Orthokeratology Investigation (COOKI) pilot study. Optom Vis Sci 2004;81(6):407-413.
17 Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, Saw SM,Chen H, Bao F, Zhao Y, Hu L, Li X, Gao R, Lu W, Du Y, Jinag Z, Yu A,Lian H, Jiang Q, Yu Y, Qu J. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology 2016;123(4):697-708.
18 Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P. Myopia control during orthokeratology lens wear in children using a novel study design.Ophthalmology 2015;122(3):620-630.
19 Lee YC, Wang JH, Chiu CJ. Effect of Orthokeratology on myopia progression: twelve-year results of a retrospective cohort study. BMC Ophthalmol 2017;17(1):243.
20 Si JK, Tang K, Bi HS, Guo DD, Guo JG, Wang XR. Orthokeratology for myopia control: a meta-analysis. Optom Vis Sci 2015;92(3):252-257.
21 Lin HJ, Wan L, Tsai FJ, Tsai YY, Chen LA, Tsai AL, Huang YC.Overnight orthokeratology is comparable with atropine in controlling myopia. BMC Ophthalmol 2014;14:40.
22 Chan B, Cho P, Cheung SW. Orthokeratology practice in children in a university clinic in Hong Kong. Clin Exp Optom 2008;91(5):453-460.
23 Regatieri CV, Branchini L, Fujimoto JG, Duker JS. Choroidal imaging using spectral-domain optical coherence tomography. Retina 2012;32(5):865-876.
24 Hua R, Liu L, Li C, Chen L. Evaluation of the effects of photodynamic therapy on chronic central serous chorioretinopathy based on the mean choroidal thickness and the lumen area of abnormal choroidal vessels.Photodiagnosis Photodyn Ther 2014;11(4):519-525.
25 Mwanza JC, Sayyad FE, Banitt MR, Budenz DL. Effect of pupil dilation on macular choroidal thickness measured with spectral domain optical coherence tomography in normal and glaucomatous eyes. Int Ophthalmol 2013;33(4):335-341.
26 Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems.Ophthalmology 2012;119(1):119-123.
27 Liu X, Shen M, Huang S, Leng L, Zhu D, Lu F. Repeatability and reproducibility of eight macular intra-retinal layer thicknesses determined by an automated segmentation algorithm using two SD-OCT instruments.PLoS One 2014;9(2):e87996.
28 Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 2009;147(5):811-815.
29 Huynh E, Chandrasekera E, Bukowska D, McLenachan S, Mackey DA, Chen FK. Past, Present, and Future Concepts of the Choroidal Scleral Interface Morphology on Optical Coherence Tomography. Asia Pac J Ophthalmol (Phila) 2017;6(1):94-103.
30 Tan CS, Cheong KX, Lim LW, Li KZ. Topographic variation of choroidal and retinal thicknesses at the macula in healthy adults. Br J Ophthalmol 2014;98(3):339-344.
31 May CA. Non-vascular smooth muscle cells in the human choroid:distribution, development and further characterization. J Anat 2005;207(4):381-390.
32 Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res 2010;29(2):144-168.
33 Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy: Bruch’s membrane changes and photoreceptor loss.Ophthalmology 2000;107(2):334-343.
34 Lee K, Lee J, Lee CS, Park SY, Lee SC, Lee T. Topographical variation of macular choroidal thickness with myopia. Acta Ophthalmol 2015;93(6):e469-e474.
35 Gardner DJ, Walline JJ, Mutti DO. Choroidal Thickness and Peripheral Myopic Defocus during Orthokeratology. Optom Vis Sci 2015;92(5):579-588.
36 Bozkurt B, Irkeç M, Gedik S, Orhan M, Erdener U, Tatlipinar S,Karaagaoglu E. Effect of peripapillary chorioretinal atrophy on GDx parametersin patients with degenerative myopia. Clin Experiment Ophthalmol 2002;30(6):411-414.
37 Bozkurt B, Irkec M, Gedik S, Orhan M, Erdener U, Tatlipinar S,Karaagaoglu E. Choroidal vascular analysis in myopic eyes: evidence of foveal medium vessel layer thinning. Int J Retina Vitreous 2017;3:28.