Table 1 Baseline characteristics in 57 C/HCRVO patients with and without OH associated
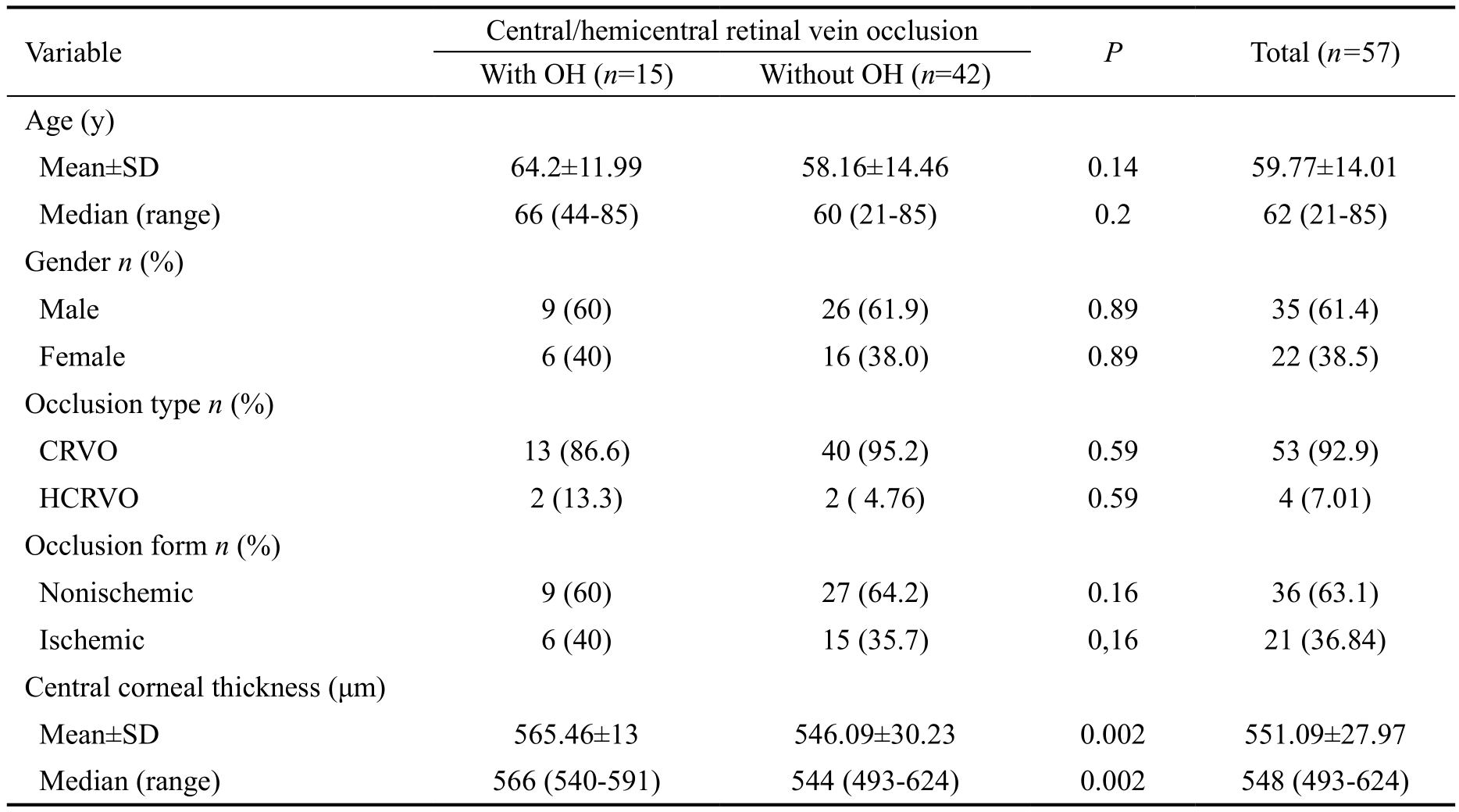
OH: Ocular hypertension; CRVO: Central retinal vein occlusion; HCRVO: Hemicentral retinal vein occlusion.
Dan Călugăru1, Mihai Călugăru1, Ştefan Ţălu2
1Department of Ophthalmology, University of Medicine, Cluj-Napoca 400467, Romania
2Department of AET Discipline of Descriptive Geometry and Engineering Graphics, Faculty of Mechanical Engineering,Technical University of Cluj-Napoca, Cluj-Napoca 400012,Romania
The term “ocular hypertension (OH)” is used when the intraocular pressure (IOP) without treatment is >21 mm Hg in the absence of well-documented and reproducible aquired glaucomatous damage, detected by standard clinical tests,in at least 3 succesive measurements. Increased IOP may be correlated with central/hemicentral retinal vein occlusion(C/HCRVO) in multiple ways. OH is sometimes associated with retinal vein occlusion, particularly in patients with systemic arterial hypertension, hypercholesterolemia or obesity[1-2], but OH has not been proven to be a causal factor of retinal vein occlusion[1]. Additionally OH has been recognized to be the most important factor in the development of openangle glaucoma, and is the only factor that can be in fluenced by medication or surgery[3]. In contrast, central retinal vein occlusion (CRVO) can cause secondary OH, such as that encountered in neovascular glaucoma or nonrubeotic angleclosure glaucoma. Barnett et al[4]found that the 10-year cumulative incidence of retinal vein occlusions in patients with OH was 2.1% in nontreated cases and 1.4% in treated cases; however, the difference between these groups was nonsignificant. When unilateral C/HCRVO is associated with OH, there is a risk of the development of a visual field and/or optic disc glaucomatous lesion in one eye and of venous occlusion in the fellow eye[5].
The aim of this study was to prospectively evaluate the cumulative prevalence and management of OH in patients with acute unilateral C/HCRVOs over the course of 3y.
Patient PopulationThe study was carried out in 57 patients with unilateral acute C/HCRVOs in the Eye Clinic of the University of Medicine Cluj-Napoca/Romania. Written informed consent according to the tenets of the Declaration of Helsinki was obtained from each patient enrolled. Approval for the study was received from the Institutional Review Board/Ethics Committee of the University. The study included patients diagnosed with both CRVO and HCRVO, as the two occlusion types are pathogenetically similar. In cases of CRVO, the only existing central retinal vein trunk within the optic nerve is involved, whereas patients with HCRVO have two central retinal vein trunks as a congenital anomaly, and develop an occlusion in only one of them.
The patients enrolled in the study had a unilateral CRVO or HCRVO with a duration of symptoms of venous occlusive event of ≤1mo. Exclusion criteria included prior treatment(filtering surgery, corneal transplantation, pars plana vitrectomy, or photocoagulation), aphakia or pseudophakia,and any vascular retinal disorders in the study eye or agerelated macular degeneration (i.e., drusen, geographic atrophy,and neovascular form) in any of the 2 eyes.
Patient ExaminationAll patients underwent a comprehensive ophthalmological examination of both eyes. Most of the tests performed [determination of the best corrected visual acuity(BCVA) score, perimetry, biomicroscopy, gonioscopy, ocular fundus examination and measurement of the IOP] were carried out at each follow-up visits (every 2mo during the first year and every 6mo thereafter for the next 2y). Optical coherence tomography, fluorecein angiography and stereoscopic photography of the optic disc were done every 6mo during the whole follow-up period.
Central/hemicentral Retinal Vein Occlusion ClassificationC/HCRVOs were divided into two groups: nonischemic and ischemic forms. The eligibility criteria for acute nonischemic C/HCRVOs were as follows[6]: a BCVA score >20/400 Snellen equivalent; normal peripheral visual field with or without relative central scotoma; mild to moderate intraretinal hemorrhages and venous tortuosity involving 4 (CRVO) or 2 (HCRVO) retinal quadrants; rare (≤4), if any, cotton wool spots; perfused retinal capillaries or small and very limited focal retinal capillary dropouts (<10 disc areas); and optic disc edema and varied grades of macular edema[7-9]. The inclusion criteria for the ischemic type of acute C/HCRVOs were determined based on the angiography result[10]. In cases with angiographyically clear evidence of retinal capillary nonperfusion zones, the criteria included 10 or more disc areas of nonperfusion. If intraretinal hemorrhages prevented a clear angiographic evaluation of retinal capillary nonperfusion, the following parameters were considered: a BCVA score ≤20/400 Snellen equivalent; ability to see ≤V/4e isopter based on the Goldmann perimeter; the presence of relative afferent pupillary defects in patients with a normal fellow eye; extensive ocular fundus changes [striking amount of hemorrhages, venous tortuosity, cotton wool spots (>5), disc and macular edema];and an IOP reduction in the occluded eye of ≥4 mm Hg compared with the congener eye. An eye was classified as having ischemic C/HCRVO by the presence of at least 4 of these 5 parameters[11].
TreatmentOH associated with C/HCRVOs in patients showing a score >5% for the risk of conversion of OH to primary open angle glaucoma (POAG) was treated with available OH medication.The treatment aimed for a decrease in IOP to <21 mm Hg with a >22% reduction from the initial IOP values.
Diagnostic CriteriaOH associated with unilateral C/HCRVOs(investigation of the contralateral uninvolved eye): 1) IOP without treatment >21 mm Hg in at least 3 successive measurements;2) normal visual field (defined as a mean deviation and pattern standard deviation within 95% confidence limits and a glaucoma hemifield test result within normal limits); 3) normal optic disc [defined as an round or slightly oval structure measuring 1.5 mm horizontally and 1.75 mm vertically with a cup-shaped depression (the physiologic cup) located slightly temporal to its geometric center and a neuroretinal rim with a relatively uniform width and a color that ranged from orange to pink]; the size of the cup-to-disc ratio was judged taking into account the optic disc diameter; 4) open anterior chamber angle without mesodermal tissue or neovascularization (NV);5) no history of attacks of intermittent angle-closure glaucoma;6) no anamnestic information or obvious signs of systemic or local causes of increased IOP, such as ocular trauma, use of steroids or pigment dispersion; 7) clear ocular media; 8) normal retinal nerve fiber layer without localized or diffuse defects.
Functional and reproducible glaucomatous lesion (perimetric progression). Worsening of the visual field was reached when at least one of the following criteria was met[12]: 1) three or more horizontally or vertically adjacent points that differ ≥5 decibels (dB) from baseline; 2) two or more horizontally or vertically adjacent points that differ ≥10 dB from baseline;3) difference of ≥10 dB across nasal horizontal meridian at≥2 adjacent points. The defect had to be reproducible and documented in 3 reliable, abnormal, and consecutive visual fields having the same test location and same abnormality indices. The time between the first and second abnormal visual field examinations was 6mo, with the third test performed between 1d to 8wk later.
Structural, reproducible glaucomatous damage (morphologic progression)[13-15]: 1) visually recognizable, progressive(documented on stereophotographs) narrowing of the neuroretinal rim area (localized or diffuse), compared with the baseline stereoscopic optic disc slides; 2) change in the documented cup-to-disc ratio ≥0.2; 3) newly registered asymmetry in the cup-to-disc ratio size (vertical and/or horizontal) of the two eyes ≥0.2; 4) the clinically significant reproducibleworsening of the optic disc had to be documented by two sets of consecutive optic disc slides; 5) occurrence of diffuse or localized defects in the retinal nerve fiber layer.
Table 1 Baseline characteristics in 57 C/HCRVO patients with and without OH associated

OH: Ocular hypertension; CRVO: Central retinal vein occlusion; HCRVO: Hemicentral retinal vein occlusion.
Assessment of the risk profiles (individualized risk of glaucoma) of the patients for conversion from OH to POAG for the subsequent 5y, conducted using the S.T.A.R.II computer system[16-17], with determination of the risk score as a percentage, as follows: 1) low risk (<5%); 2) moderate risk(5%-15%); 3) high risk (>15%).
Main Outcome MeasurementsThe primary outcome measurement was the cumulative prevalence of OH in patients with acute C/HCRVOs. The secondary outcome measurement was the effectiveness of treatment, which was assessed by the cumulative prevalence of conversion from OH to POAG.
Statistical AnalysisDistribution normality was tested using the Kolmogorov-Smirnov test. The continuous variables were tested using Student’s t-tests (normally distributed data), and both the Man-Whitney U and Wilcoxon tests were used for nonparametric distributions. Categorical data were analyzed using the Pearson Chi-square test and Fisher’s exact test.Cumulative prevalence calculations were performed with the Kaplan-Meier estimation method. A P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software (version 16) and Epiinfo 2000.
The baseline characteristics of the 57 patients with acute C/HCRVOs are shown in Table 1. Fifteen patients had OH associated with C/HCRVOs. The differences in the average age, sex, type, and clinical form of the occlusions in patients with and without OH were not statistically significant. The central corneal thickness(CCT) of the occlusion patients with OH was significantly greater (565.46±13 μm) than the CCT of those without OH(546.09±30.23 μm; P=0.002). Particularities associated with the 15 patients with OH are summarized in Table 2. For 12 of these patients, OH was detected at the initiation of the study,and in 3 cases (cases 2, 8, and 11), OH was identified at 4,18, and 12mo, respectively, into the follow-up period. The cumulative prevalence of OH in patients with C/HCRVOs was 29.4% (95% confidence interval, 16.9-41.9; Figure 1),and the risk of OH conversion to POAG for the 5 subsequent years ranged from 6.8% to 25.5% (Table 2), with a mean value of 11.72%±5.4% (Table 3). Six OH patients (cases 3-7, 11)were treated with 0.03% bimatoprost (Lumigan, Allergan,Inc., Irvine, CA, USA; Table 2). Because the IOP values were elevated, and additional risk factors were present, we used available fixed combinations to initiate therapy (first therapeutic line), namely, 0.5% timolol/0.03% bimatoprost(FCTB; Ganfort, Allergan, Inc., Irvine, CA, USA) in 4 patients(cases 1, 8, 13, 15), and 0.5% timolol/2% dorzolamide (FCTD;Cosopt, Merck and Co, Inc., Whitehouse Station, NJ, USA)in 5 patients (cases 2, 9, 10, 12, 14). The IOP significantly decreased from 25.67±2.16 mm Hg to 18.73±2.96 mm Hg(P<0.0001) by the end of the study (Table 3). The percentage of IOP reduction compared to the baseline values was 27.03%.None of the OH patients converted to POAG during the 3-year follow-up period. Six C/HCRVO patients without OH associated were lost to follow-up, i.e., three patients with nonischemic C/HCRVOs were lost at 8, 12 and 24mo, 1 ischemic CRVO patient withdrew from the study at month 10 of follow-up and two patients with ischemic C/HCRVOs died at months 12 and 30, of the study.
Table 2 Characteristics of 15 patients with C/HCRVOs associated with OH

CRVO: Central retinal vein occlusion; HCRVO: Hemicentral retinal vein occlusion; IOP: Intraocular pressure; PSD: Pattern standard deviation;PEX: Pseudoexfoliation; c/d: Cup-to-disc ratio; ODH: Optic disc hemorrhage; Ac/d: Asymmetry of the cup-to-disc ratios between the two eyes;FCTB: Fixed combination timolol/bimatoprost; B: Bimatoprost; FCTD: Fixed combination timolol/dorzolamide.
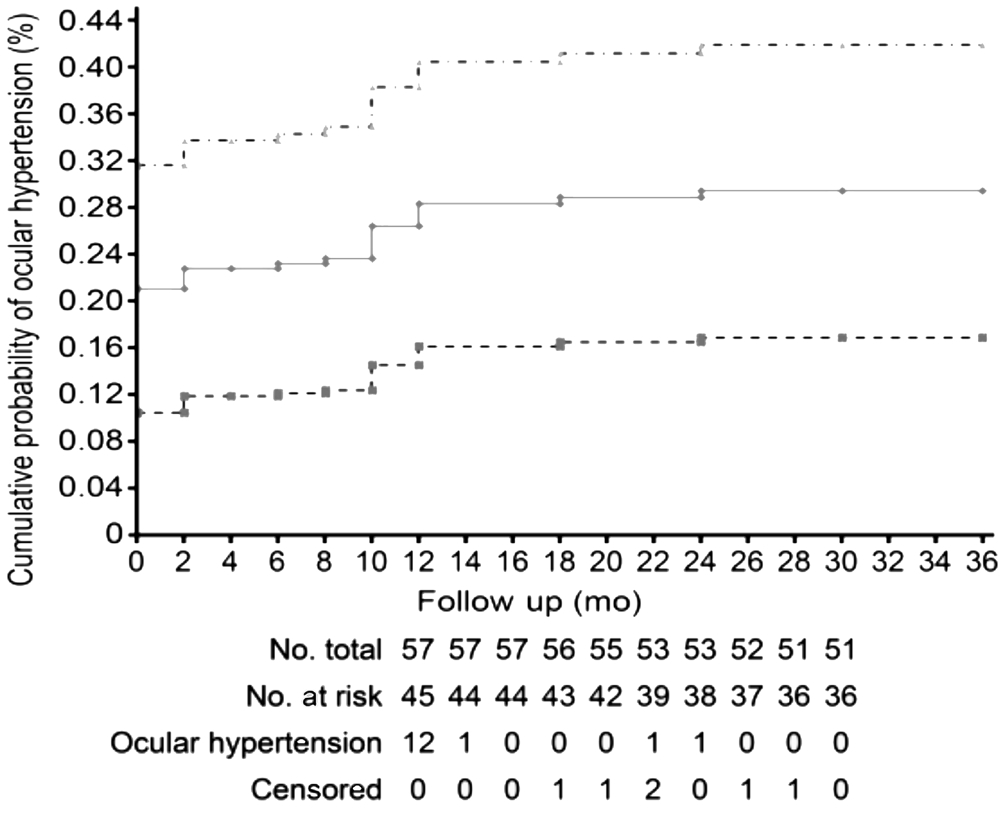
Figure 1 The Kaplan-Meier estimate of cumulative prevalence of OH along the entire course of the study in 57 patients with acute unilateral C/HCRVOs Patients at risk are those who did not present with OH at the beginning of the study period. Patients who were lost before the end of the study, were censored from the last completed visit and those who died, were censored at their date of death. The dotted lines represent the upper and lower bounds of the 95% confidence interval.
In our study, the cumulative prevalence of OH in C/HCRVO patients was much greater than that within the general population (4%-7% of the USA population older than 40y; 5%of the predominantly European population ≥50y)[16]; however,the prevalence was different from that reported by Hayreh et al (16.2%)[5]. The inconsistency between our data and those reported by Hayreh et al[5]was likely due to differences in the eligibility criteria. In contrast to the study of Hayreh et al[5], which included among patients with OH associated with venous occlusions only those with normal optic discs and normal visual fields, but which excluded cases with pseudoexfoliation (PEX), our study enrolled patients with optic disc hemorrhages and those with PEX.
The risk profiles of individual patients, which we calculated using the Ocular Hypertension Treatment Study (OHTS)/European Glaucoma Prevention Study (EGPS) risk computer program[16-17]were essential for the management of our patients.Because, the risk score for the conversion of OH to POAG was>5% in our series, we applied OH therapy in all patients with OH and associated C/HCRVOs. The targets of treatment were an IOP decrease to values <21 mm Hg and an average percent IOP reduction of >22%, compared to the baseline values.We chose the IOP value of <21 mm Hg because the value of≤24 mm Hg, that was established by the OHTS as a target for treated patients, was not sufficient to prevent progression of OH to POAG in all of the patients[13]. Using this value, only 53.68% of the cases exhibited a decreased risk of progression from OH to POAG. When analyzing the percentage of IOP reduction due to treatment compared to baseline values, we considered the results of the OHTS[13-15]and the EGPS[18]. In both studies, a reduction in IOP of approximately 22% (22.5%in the OHTS[13]and between 15% and 21% in the EGPS[18])was insufficient to prevent conversion to POAG. Thus, in the OHTS[13], which included participants at a greater risk of conversion (healthy Caucasian, African, American, and Hispanic volunteers aged between 40y and 80y; 1/3 were athigh-risk, and 2/3 were at moderate risk), the conversion to POAG in treated patients decreased significantly by 53.68%[15].In contrast, in the EGPS[18], which enrolled patients at moderate risk of conversion (the general European Caucasian population aged ≥30y), the risk of conversion insignificantly decreased by 4.96% in treated patients, when only considering the efficiency criteria and insignificantly decreased by 16.4%,when considering both the efficiency and safety criteria. The EGPS[18]failed to detect a statistically significant protective effect of the medical treatment. For these reasons, we chose a percentage greater than 22% as the target for the average reduction in IOP upon ocular hypotensive treatment.
Table 3 Descriptive and discriminating statistics of 15 cases with C/HCRVOs associated with OH

IOP: Intraocular pressure; PSD: Pattern standard deviation; Initial vs final IOP: P<0.0001 for both the mean and median; Initial vs final PSD:P=0.52 for the mean and P=0.9 for the median.
We included in our series 2 patients (cases 1 and 15) with a cup-to-disc ratio of 0.6 and one patient (case 9) with asymmetry of the cup-to-disc ratio between the two eyes (Table 2). The topography of the optic discs in our series could be explained by greater than normal of the optic disc diameters. Thus, two of our patients with a cup-to-disc ratio of 0.6 (cases 1 and 15) had disc diameters of 2.31 mm and 2.16 mm, respectively. Another patient (case 9) with asymmetric cup-to-disc ratios between the 2 eyes had a 1.67 mm disc diameter in the occlusion eye and a 2.15 mm disc diameter in the contralateral eye.
Additional risk factors, that we encountered in our series were optic disc hemorrhages (ODH) and PEX. Hemorrhages at the edge of the optic disc with a flame- or splintershaped appearance were confirmed in 4 patients (cases 2,10, 13, and 14) by clinical examination and review of disc stereophotographs (3 cases with ODH at baseline examination and 1 case with ODH occurring during the follow-up period;Table 2). The ODHs were localized in the retinal nerve fiber layer, were radially oriented and perpendicular to the edge of the optic disc, had feathered ends, and were not associated with papillary edema. The hemorrhages disappeared within 7-10mo and did not recur during the follow-up. ODHs indicate an active disease process and are frequently followed by changes in the optic disc and visual field. However, conversion to POAG did not occur in any of our cases. Budenz et al[19]reported a 13.6% cumulative incidence of POAG in OH eyes with ODHs during a 96-month follow-up. Although most OH patients with ODHs do not develop manifest POAG (86.7%),ODH, together with all of the well-known conventional risk factors, should be considered a powerful risk factor, that can predict POAG. The presence of ODHs caused us to initiate OH therapy in all patients with OH and associated C/HCRVOs.
Of 3 cases with OH and associated C/HCRVOs (cases 8, 12,and 13; Table 2) had PEX. As these patients were at highrisk for conversion, they were treated with OH medications;however, none of these patients experienced conversion to POAG. PEX is an additional, powerful and independent risk factor for conversion to POAG. A previous study showed that rate of glaucoma conversion was twice as high in the OH patients with PEX than in a control group matched for baseline IOP, age, and sex without PEX (6.3% and 3.2%, respectively)[20].PEX has been associated with a less favorable prognosis because it can cause increased IOP through the production and storage of PEX and pigment materials in the trabecular meshwork and Schlemm’s canal, with subsequent increased outflow resistance. The presence or absence of PEX, along with the predictive conventional risk factors described by the OHTS[13]must be considered, particularly because PEX glaucoma tends to progress more quickly than glaucoma without PEX. Moreover, PEX is a significant risk factor for developing retinal vein occlusion[21].
Most of the patients in our series with OH and associated C/HCRVOs presented a thicker CCT, with the average value being significantly greater (565.46±13 μm; P=0.002) than in the patients without OH (546.09±30.23 μm; Table 1). Because a thin CCT represents an independent, well-known risk factor for conversion of OH to POAG, thicker CCT could have an inverse effect, i.e. a protective effect against the development of glaucoma. The following could explain the protective effect of a thicker CCT: higher IOP values than those existing in reality, as measured by applanation tonometry, that require a more aggressive OH therapy; more rigid optic nerve architecture (including lamina cribrosa), that is less likely to develop a glaucomatous lesions; and less distensibility and elasticity of the ocular tissues.
The main limitation of our study was the lack of a control group including C/HCRVO patients for determining the cumulative prevalence of OH, as well as conversion to POAG,in the absence of OH treatment. Similarly, the observation period of 3y in our series was too short to be able to assess the conversion from OH to POAG in treated patients.
In conclusion, the increased cumulative prevalence of OH in C/HCRVO patients indicates that OH is a risk factor for the appearance of venous occlusion. Patients with OH associated with C/HCRVOs must be considered to be at high risk for conversion to POAG. Initiating treatment with ocular hypotensive medication in these patients, which serves a dualpurpose, namely, achieving a pressure value <21 mm Hg and a reduction in IOP of >22% over the initial values, prevented conversion to POAG during the 3-year follow-up.
All authors were involved in design and conduct of the study;collection, management, analysis and interpretation of the data;and preparation, review or approval of the manuscript.
Conflicts of Interest: Călugăru D,None;Călugăru M,None;Țălu S,None.
REFERENCES
1 Călugăru D, Călugăru M. Comment on: “Combination of peripheral laser photocoagulation with intravitreal bevacizumab in naive eyes with macular edema secondary to CRVO: prospective randomized study”.Eye(Lond)2016;30(11):1520-1521.
2. Călugăru D, Călugăru M. Ranibizumab versus dexamethasone implant for central retinal vein occlusion: the Ranidex study.Graefes Arch Clin Exp Ophthalmol2017:255(10):2073-2075.
3 Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E;Early Manifest Glaucoma Trial Group. Early Manifest Glaucoma Trial Group factors for glaucoma progression and the effect of treatment. The Early manifest Glaucoma Trial.Arch Ophthalmol2003;121(1):48-56.
4 Barnett EM, Fantin A, Wilson BS, Kass MA, Gordon MO; Ocular Hypertension Treatment Study Group. The incidence of retinal vein occlusion in the Ocular Hypertension Treatment Study.Ophthalmology2010;117(3):484-488.
5 Hayreh SS, Zimmerman MB, Beri M, Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion.Ophthalmology2004;111(1):133-141.
6 Călugăru D, Călugăru M. Outcomes of patients initially treated with intravitreal bevacizumab for central retinal vein occlusion: long-term follow-up.Semin Ophthalmol2018;33(3):318-319.
7 Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome.Prog Retin Eye Res2014;41:1-25.
8 Hayreh SS, Zimmerman MB, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion.Am J Ophthalmol2001;131(1):61-77.
9 Morshedi RG, Ricca AM, and Wirostko BM. Ocular hypertension following intravitreal antivascular endothelial growth factor therapy:review of the literature and possible role of nitric oxide.J Glaucoma2016;25(3):291-300.
10 Călugăru D, Călugăru M. Predictive factors for functional improvement following intravitreal bevacizumab injections after central retinal vein occlusion.Graefes Arch Clin Exp Ophthalmol2017;255(5):1043-1044.
11 Călugăru D, Călugăru M. Intravitreal bevacizumab in acute central/hemicentral retinal vein occlusions: three-year results of a prospective clinical study.J Ocul Pharmacol Ther2015;31(2):78-86.
12 Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I;European Glaucoma Prevention Study Group. The European Glaucoma Prevention Study design and baseline description of the participants.Ophthalmology2002;109(9):1612-1621.
13 Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma.Arch Ophthalmol2002;120(6):701-713.
14 Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys.Br J Ophthalmol2002;86(2):238-242.
15 Gordon MO and Kass MA. What we have learned from the ocular hypertension treatment study.Am J Ophthalmol2018;189(5):24-27.
16 Weinreb RN, Friedman DS, Fechtner RD, CioffiGA, Coleman AL,Girkin CA, Liebmann JM, Singh K, Wilson MR, Wilson R, Kannel WB.Risk assessment in the management of patients with ocular hypertension.Am J Ophthalmol2004;138(3):458-467.
17 Kass MA, Gordon MO, Gao F, Heuer DK, Higginbotham EJ,Johnson CA, Keltner JK, Miller JP, Parrish RK, Wilson MR; Ocular Hypertension Treatment Study Group. Delaying treatment of ocular hypertension: the Ocular Hypertension Treatment study.Arch Ophthalmol2010;128(3):276-287.
18 Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I;European Glaucoma Prevention Study (EGPS) Group. Results of the european glaucoma prevention study.Ophthalmology2005;112(3):366-375.
19 Budenz DL, Anderson DR, Feuer WJ, Beiser JA, Schiffman J, Parrish RK 2nd, Piltz-Seymour JR, Gordon MO, Kass MA, . Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study.Ophthalmology2006;113(12):2137-2143.
20 Grødum K, Heijl A, Bengtsson B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation.Ophthalmology2005;112(3):386-390.
21 Cursiefen C, Händel A, Schönherr U, Naumann GO. Pseudoexfoliation syndrome in patients with retinal vein branch and central vein thrombosis.Klin Monbl Augenheilkd1997;211(1):17-21.
Correspondence to:Mihai Călugăru. Department of Ophthalmology, University of Medicine, Strada Brâncoveanu 11, Cluj-Napoca 400467, Romania. mihai.calugaru@mail.dntcj.ro
Received:2017-01-11 Accepted: 2017-11-26
Abstract ● AlM: To prospectively evaluate the cumulative prevalence and the management of ocular hypertension (OH) in patients with unilateral acute central/hemicentral retinal vein occlusions (C/HCRVOs) over the course of 3y.● METHODS: The study included 57 patients with unilateral acute C/HCRVOs. All patients underwent a comprehensive ophthalmological examination of both eyes. OH associated with C/HCRVO in patients showing a score >5% for the risk of conversion to primary open angle glaucoma (POAG)was treated with OH medication. The treatment aimed for a decrease in intraocular pressure (lOP) to <21 mm Hg with a >22% reduction from the initial values. The cumulative prevalence of OH and the effectiveness of treatment assessed by the cumulative prevalence of conversion from OH to POAG, were estimated.● RESULTS: Fifteen patients had OH associated with C/HCRVOs, the cumulative prevalence of OH was 29.4%(95% confidence interval, 16.9-41.9). The mean value of the risk score of OH conversion to POAG for the 5 subsequent years was 11.7%±5.4%. The lOP significantly decreased from 25.67±2.16 mm Hg to 18.73±2.96 mm Hg.None of the OH patients converted to POAG during the follow-up period.● CONCLUSlON: The increased cumulative prevalence of OH in C/HCRVO patients indicates that OH is a risk factor for the appearance of venous occlusion. Patients with OH associated with C/HCRVO must be considered to be at high risk for conversion to POAG. Treatment with OH medications prevented conversion to POAG during the 3-year follow-up.
● KEYWORDS:ocular hypertension; acute central/hemicentral retinal vein occlusion; intraocular pressure; primary openangle glaucoma; risk factor.
DOl:10.18240/ijo.2018.07.16
Citation:Călugăru D, Călugăru M, Ț ălu Ş. Ocular hypertension in patients with central/hemicentral retinal vein occlusions: cumulative prevalence and management. Int J Ophthalmol 2018;11(7):1173-1178