In vivo biodistribution of topical low molecular weight heparin-taurocholate in a neovascularized mouse cornea
Chan Hee Moon 1 , Ji Yun Lee 1 , Eun Soon Kim 1 , Jin Hyoung Park 2 , Sang-Yeob Kim 3 , Jae Yong Kim 1 ,Hungwon Tchah 1
1 Department of Ophthalmology, Asan Medical Center,University of Ulsan College of Medicine, Seoul 05505,Republic of Korea
2 Research Institute for Biomacromolecules, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Republic of Korea
3 Department of Convergence Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505,Republic of Korea
Abstract · AlM: To investigate the ocular biodistribution and clearance of topically administered 7-taurocholic acid conjugated low-molecular weight heparin (LHT7) in a neovascularized mouse cornea using an in vivo optical imaging system.· METHODS: A total of 10 eyes of 6 to 8-week-old BALB/c mice were analyzed. Corneal neovascularization (CoNV)was induced in the inferior cornea (lC) of each animal by penetrating the stroma with two interrupted sutures. The development of CoNV was verified after one week and the area of each neovascularized region was measured. A near-infrared fluorescent probe of 20 µmol/L Cy5.5 labeled LHT7 (LHT7-Cy5.5) in 0.02 mL solution was topically instilled onto the cornea in the experimental group (n=5).Free-Cy5.5 of 20 µmol/L in 0.02 mL was instilled in the control group (n=5). In vivo optical images were obtained before instillation and 5min, 2, 4, and 6h after instillation.The intensities were separately measured at the superior cornea (SC) and the lC.· RESULTS: The mean CoNV areas were 1.97±0.17 mm2 and 1.92±0.96 mm2 in the experimental and control groups,respectively (P=0.832). The SC remained normal in all 10 subject animals. The lC intensity of the LHT7-Cy5.5 was greater than the SC intensity at 5min (P=0.038), 2h (P=0.041),and 4h (P=0.041) after application. The lC intensity fell to less than half of its initial value (42.9%±8.6%) at 6h in the experimental group. ln the control mice, here were no significant differences in the free-Cy5.5 intensity between the lC and SC.· CONCLUSlON: Topically administered LHT7 shows a high biodistribution in CoNV areas for 4h and should be reapplied accordingly to maintain its effects. In vivo optical imaging can be a useful tool for evaluating the ocular biodistribution of a drug in an animal model.
· KEYWORDS: corneal neovascularization; in vivo optical imaging; low-molecular weight heparin; ocular biodistribution
INTRODUCTION
The cornea is normally a transparent avascular tissue.A disequilibrium between pro- and anti-angiogenic stimuli, due to various insults including ischemia, infection,inflammation and trauma causes corneal neovascularization(CoNV) [1] . Several established therapies such as steroids, nonsteroidal anti-inflammatory drugs, cyclosporin A, corneal limbal cell transplantation, and argon laser photocoagulation are available to treat CoNV. However, there remains no clear consensus on the best treatment [2] .
Heparin has been used as an anti-coagulant for several decades.Recent studies have shown that it has anti-angiogenic effects in addition to anti-inflammatory and immunomodulating effects [3] . Chemically modified heparin derivatives have also been reported to show advanced anti-angiogenic activity with decreased anti-coagulation effects and less complications due to bleeding [4-5] . A modified heparin derivative, 7-taurocholic acid conjugated low-molecular weight heparin (LHT7) has been proven to be a potent anti-angiogenic effects [6] . Several previous studies have also demonstrated that low-molecular weight heparin (LMWH) inhibits CoNV in experimental animal models [7-10] . However, the route of administration and dosage of heparin in the treatment of CoNV have varied from study to study. In addition the ocular pharmacokinetics and biodistribution of administered heparin is not well documented.A recently introduced in vivo optical imaging system has provided valuable information on the biodistribution, stability,and clearance profiles of a variety of pharmaceutical agent in a non-invasive manner. Notably, the in vivo fate of near-infrared(NIR) fluorescent-probe-labeled substances can be easily monitored using this system [11-12] .
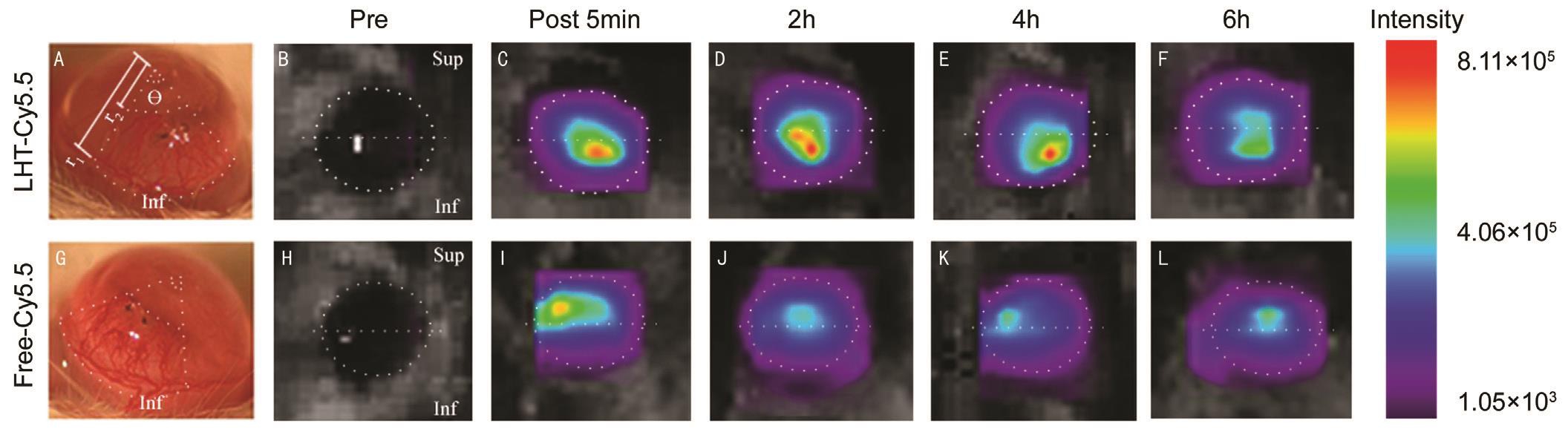
Figure 1 Representative microscopic photographs and in vivo optical images CoNV developed in the ICs of the mice one week after suturing, at which time the annular sectoral neovascularized area was measured (A, G). In the pre-scan, autofluorescence was detected, and the region of interest was determined (white dotted line, B, H). LHT7-Cy5.5 showed a high distribution in the neovascularized IC for 4h after its topical application (C-E), which was reduced at 6h (F). Free-Cy5.5 control showed an even distribution in both the SC and IC regardless of neovascularization (I-L). Ɵ : Central angle in radians; r 1 : Radius of the corneal center to the CoNV starting point; r 2 : Radius of the corneal center to the CoNV end point.
In our current study, we assessed the ocular biodistribution and clearance of topically administered LHT7 using an in vivo optical imaging system in a mouse model of neovascularized cornea.
MATERIALS AND METHODS
Animals Ten healthy eyes from 6 to 8-week-old BALB/c mice weighing 20-30 g were used in the experiments. The mice were maintained in individual cages under standard conditions and handled in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The experimental protocols of the present study were approved by the Institutional Animal Care and Use Committee of Asan Medical Center.
Corneal Neovascularization Mouse Model For intra-animal comparisons, CoNV was only induced in the inferior cornea(IC). These procedures were performed under anesthesia via an intraperitoneal injection of zolazepam (80 mg/kg) and xylazine hydrochloride (10 mg/kg). Proparacaine hydrochloride at a 0.5% concentration (Alcaine; Alcon-Couvreur, Puurs,Belgium) was also topically applied. For suture-induced CoNV, marks were created in the IC at 1 mm from the limbus.Two interrupted sutures were placed through the epithelium and stroma with 10-0 nylon (AA-2626; Sharpoint, Surgical Specialties Corp., Vancouver, Canada). These sutures did not penetrate the endothelial layer. The corneal surface was then rinsed with 10 mL balanced salt solution (Alcon Laboratories,Fort Worth, TX, USA).The development of CoNV was evaluated for one week after the suturing procedure. Annular sectorial areas of the CoNV(Figure 1) were then measured as follows

where Ɵ is the central angle (radians), r 1 is the radius of the corneal center to CoNV starting point and r 2 is the radius of the corneal center to CoNV end point.
Taurocholic Acid-conjugated Low-molecular Weight Heparin LHT7 was synthesized according to a previously described method [13] . Briefly, a primary amine was introduced into 4-hydroxyl sodium taurocholate and cholic acid by activating the hydroxyl group using 4 nitrophenyl chloroformate(Sigma-Aldrich, St. Louis, MO, USA) in the presence of triethylamine (Sigma-Aldrich), followed by the addition of excessive ethylenediamine (Sigma-Aldrich). The carboxylic group of the cholic acid was protected in advance.Ethylenediamine-introduced sodium taurocholate was conjugated onto LMWH (Fraxiparin; Glaxo Smith Kline,Genval, Belgium) via its carboxylate groups through the 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC)/N-hydroxysuccinimide (NHS) coupling method to produce LHT7.
The NIR fluorescence cyanine (Cy) dye, Cy5.5, was used as the fluorescent probe due to its ability to penetrate tissues, its small size, good aqueous solubility, pH insensitivity in the 3-10 range, and low non-specific binding [14] . Cy5.5 NHS ester was purchased from the Lumiprobe Corporation (Hallandale Beach, FL, USA) and used to synthesize Cy5.5 labeled LHT7.LHT7 was weighed and dissolved in sterile water by ultrasonic agitation prior to use.
In vivo Optical Imaging An in vivo optical imaging system(Optix MX3 Molecular Imaging System, Advanced Research Technology, Inc., Montreal, Canada) was used to determine the ocular biodistribution of LHT7 in the mouse. A laser light source of a 670 nm wavelength and resolution power of 0.5 mm was used for image acquisition.
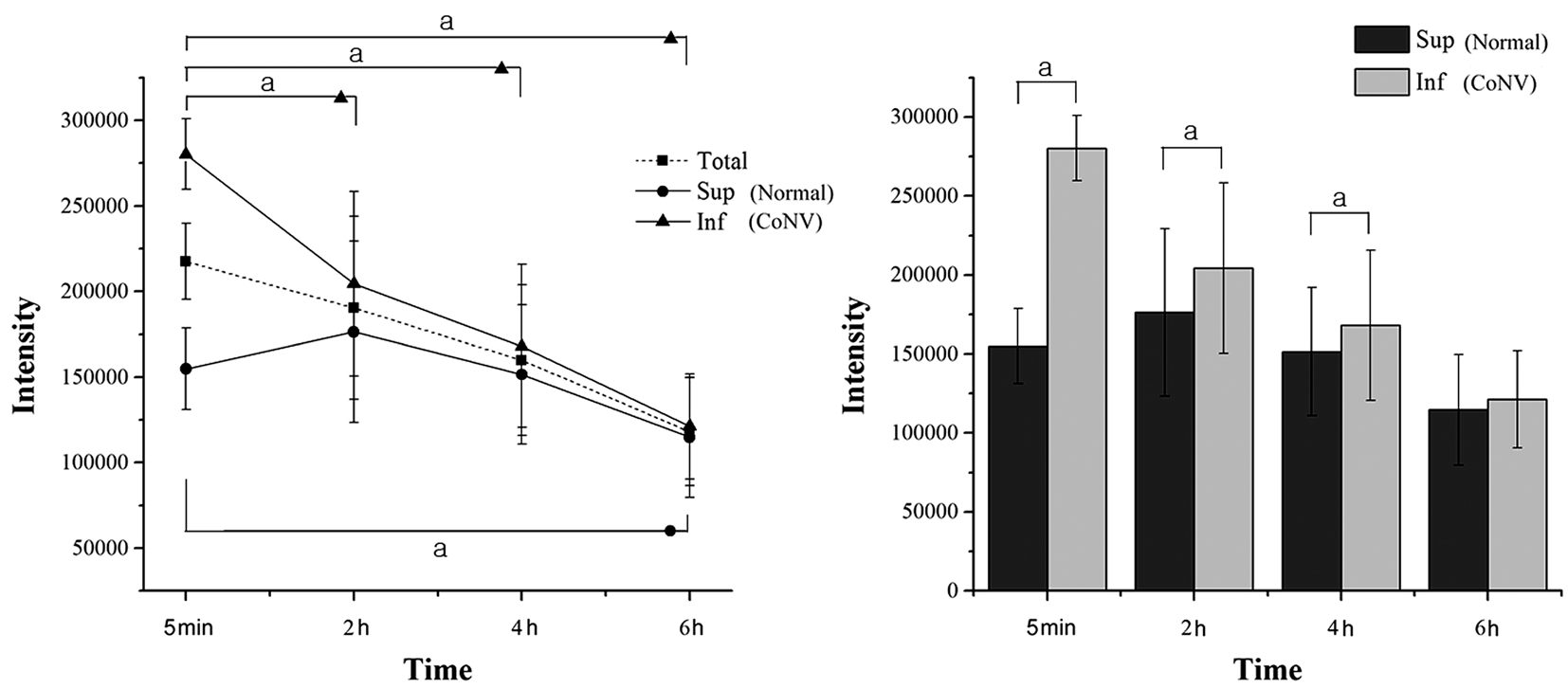
Figure 2 Measurement of LHT7-Cy5.5 fluorescent intensities a P <0.05 tested by Wilcoxon signed-ranks test.
Ten mice were randomly divided into two groups: 20 µmol/L Cy5.5 labeled LHT7 (LHT7-Cy5.5) in 0.02 mL solution was topically instilled to the cornea in experimental group ( n =5)and 20 µmol/L free-Cy5.5 in 0.02 mL solution was instilled in control group ( n =5). In vivo optical images were obtained before instillation and 5min, 2, 4, and 6h after instillation. The cornea was divided into a superior and inferior area based on the horizontal midline. Intensities were measured in the superior cornea (SC), which was not a neovascularized area,in the IC which was a neovascularized area, and over the total corneal area (Figure 1). The cornea was designated as the region of interest (ROI). The relative mean fluorescence intensity for each ROI was calculated by subtracting the mean intensity of the corresponding ROI on a pre-scan and was then plotted as a function of time. The intensity of Cy5.5 was analyzed using Optix MX3 Optiview software (USA).
Statistical Analysis The Mann-Whitney U test was performed to evaluate differences in the CoNV area between the experimental and control groups. Friedman tests were used to assess the statistical significance of changes in intensity between the different time points within the groups, and posthoc analyses were conducted using the Wilcoxon signed rank test. Differences in the intensities between the SC and IC at each time point within groups were also compared using the Wilcoxon signed rank test. Statistical analyses were conducted using SPSS Statistics version 21 software (IBM Corporation,Somers, NY, USA). All tests were two-tailed and P <0.05 were considered statistically significant.
RESULTS
Corneal Neovascularization One week after suture, there were CoNVs in the ICs. The mean CoNV area was 1.97±0.17 mm 2 in the experimental group and 1.92±0.96 mm 2 in the control group.
The differences in CoNV areas between the experimental and control groups were not significant ( P =0.832). All of the SCs remained normal in both groups.
Biodistribution of Cy5.5 labeled LHT7 LHT7-Cy5.5 showed a higher accumulation in the neovascularized ICs than in the normal SCs. No autofluorescence was detected in the pre-scans (Figure 1). The IC intensity of LHT7-Cy5.5 was greater than the SC intensity of this molecule at 5min( P =0.038), 2h ( P =0.041) and 4h ( P =0.041) after its topical application. The IC intensity of LHT7-Cy5.5 gradually decreased, to a significantly reduced level at 2h ( P =0.041), 4h( P =0.041), and 6h ( P =0.041) compared to that at 5min posttreatment. The IC intensity of LHT7-Cy5.5 at 6h was less than half of that at 5min after instillation (42.9%±8.6%). The difference in intensity between IC and SC was not significant at 6h ( P =0.684). The SC intensity of LHT-Cy5.5 was steady from 5min to 4h after instillation. The differences in SC intensity between 5min and 2h ( P =0.221), and 4h ( P =0.683)were not significant. The SC intensity of LHT7-Cy5.5 was significantly lower at 6h than at 5min ( P =0.042; Figure 2).
Biodistribution of Free-Cy5.5 The free-Cy5.5 control agent showed an analogous distribution and clearance pattern between the neovascularized IC and the normal SC.Autofluorescence was again not detected on the pre-scans(Figure 1). The intensities of free-Cy5.5 in IC and SC were not significantly different at 5min ( P =0.461), 2h ( P =0.593),4h ( P =0.269) or 6h ( P =0.713) after instillation. Free-Cy5.5 was maintained at a similar intensity from 5min to 4h in both the IC and SC in these control animals. The differences in IC intensity between 5min, 2h ( P =0.269), and 4h ( P =0.276) postapplication were not significant. Changes in the SC intensity between the 5min, 2h ( P =0.713), and 4h ( P =0.461) time points were also not significant. After 6h post-treatment, the free-Cy5.5 intensity was significantly reduced in both the IC ( P =0.038) and SC ( P =0.041; Figure 3).
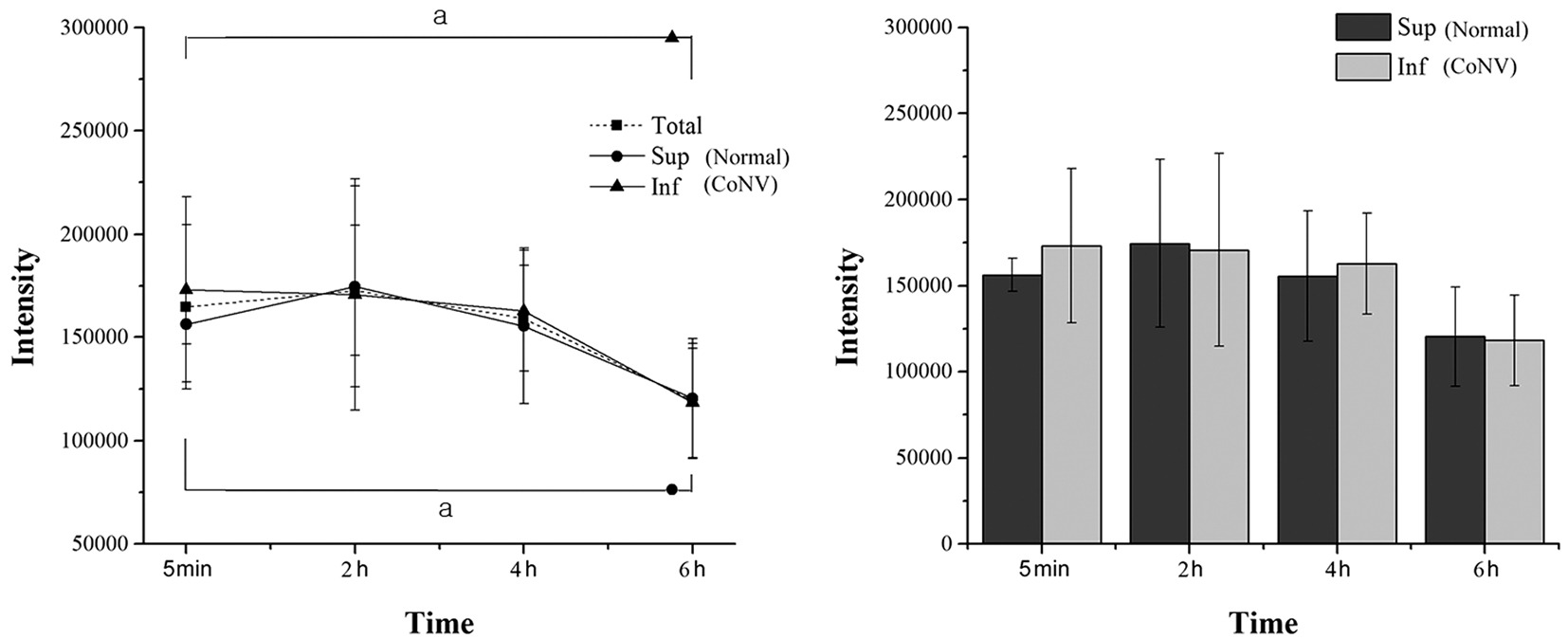
Figure 3 Measurements of Free-Cy5.5 fluorescent intensities a P <0.05 tested by Wilcoxon signed-ranks test.
DISCUSSION
In recent years, biodistribution analyses of pharmaceutical compounds in preclinical animal models have become an integral part of drug development [15] . In our previous study, we reported that LHT7 efficiently inhibits CoNV in an experimental animal model [8] . In our present study, we investigated the ocular biodistribution of topically applied LHT7 using in vivo optical imaging in a mouse model of CoNV.Traditionally, to evaluate the tissue distribution of a biochemical substance, tissue histology has been necessary. Subsets of animals would thus be sacrificed at different time points to determine the temporal patterns of distributions and levels of the substance. This approach is therefore costly and lengthy;furthermore, it requires a large number of animals. It also decreases the statistical power of the experiment because each animal is only measured once and cannot be its own control [16] . In vivo optical imaging has offered an opportunity to measure the biodistribution of a pharmaceutical at multiple time points,non-destructively, and reduce the variance associated with inter-animal comparisons. It also makes it possible to observe the physiology of an animal in real-time rather than relying on a static post-mortem histology. Many experimental and clinical applications of optical imaging have been explored [17-18] .Moreover, this field have continued to evolve. Novel types of luminescent probes including nanocluster material have been introduced, which are have high fluorescence, good photostability, non-toxicity and excellent biocompatibility [19] .Deep optical imaging of tissue has been reported using the second and third NIR spectral windows to reduce scattering,minimize absorption then enhance image quality [20] . However,to the best of our knowledge, our current study is the first to use in vivo optical imaging for ocular biodistributional assessment. In vivo optical imaging must address a trade-off between the depth of targeted tissues and the resolution of the image produced. The eyes are exposed organ, therefore they have properties suitable for in vivo optical imaging. In this study,we utilized the relatively simple fluorescent probe, Cy5.5, and did not applicate additional steps otherwise conventional in vivo optical imaging process. Nevertheless, acquired images were discernible and measurable and we found that it is usable for biodistribution evaluations of a pharmaceutical agent. To verify the usefulness of in vivo optical imaging for ocular biodistribution in various material and optimize the image quality, further studies will be needed.
Topically-administered LHT7 showed a high abundance at the CoNV areas of the subject mice for 4h but this level dropped by more than 50% at 6h after instillation. In contrast,LHT7 demonstrated a low distribution in normal corneas,and free cyanine also produced comparably low signals in both neovascularized and normal mouse corneas, without a difference. Vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2) play significant roles in neovascularization including CoNV, and platelet-derived growth factor B (PDGF-B) is a key factor in stabilizing the primitive vascular network by recruiting mural cells to the endothelium [21-22] . LHT7 has been found to inhibit multiple stages of angiogenesis by blocking VEGF, FGF2, and PDGF-B [6] . Undoubtedly, definite clinical effect of LHT7 on CoNV regression have to be determined using gross manner.In our other experimental study, we evidenced that topical administration of LHT7 has an effect on CoNV regression and prevention [9] . The high distribution of LHT in CoNV areas reinforce the assumption that LHT7 interacts with the angiogenic factors. With regard to the half-life, our present results suggest that topically administered LHT7 maintains its effects for at least 4h, and that additional application would therefore be needed every 4 to 6h.
There were a number of limitations to the present study. We did not establish a therapeutic window, and did not evaluate the relationship between the biodistribution level and pharmacological effects of LHT7. Further investigations will be needed to determine the correlation between the clinical effectiveness and biodistributional degree.
In conclusion, topically-administered LHT7 shows a high biodistribution in CoNV areas in the mouse for 4h after its topical application at the cornea. Additional applications would thus be required every 4 to 6h to maintain its distribution level. In vivo optical imaging with an NIR probe is a useful approach to evaluating the ocular biodistribution of pharmacological agents in an animal model.
ACKNOWLEDGEMENTS
We would like to thank Dr. Youngro Byun, College of Pharmacy,Seoul National University, Seoul, Republic of Korea who kindly provided taurocholic acid conjugated low-molecular weight heparin .
Authors’ contributions: All named authors meet the International Committee of Medical Journal Editors (ICMJE)criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Foundation: Supported by a grant (No.2016-7026) from the Asan Institute for Life Science, Seoul, Republic of Korea.
Conflicts of Interest: Moon CH, None; Lee JY, None; Kim ES, None; Park JH, None; Kim SY, None; Kim JY, None; Tchah H, None.
REFERENCES
1 Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006;104:264-302.
2 Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol 2001;12(4):242-249.
3 Lever R, Page CP. Novel drug development opportunities for heparin. Nat Rev Drug Discov 2002;1(2):140-148.
4 Alam F, Hwang SR, Al-Hilal TA, Chung SW, Kim HS, Kang BH, Zhang HS, Shin SH, Lee JY, Kang MS, Kwon GH, Jeon OC, Kim SY, Byun Y.Safety studies on intravenous infusion of a potent angiogenesis inhibitor:taurocholate-conjugated low molecular weight heparin derivative LHT7 in preclinical models. Drug Dev Ind Pharm 2016;42(8):1247-1257.
5 Park K, Lee GY, Kim YS, Yu M, Park RW, Kim IS, Kim SY, Byun Y. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J Control Release 2006;114(3):300-306.
6 Chung SW, Bae SM, Lee M, Al-Hilal TA, Lee CK, Kim JK, Kim IS,Kim SY, Byun Y. LHT7, a chemically modified heparin, inhibits multiple stages of angiogenesis by blocking VEGF, FGF2 and PDGF-B signaling pathways. Biomaterials 2015;37:271-278.
7 Peyman GA, Kazi AA, Riazi-Esfahani M, Aydin E, Kivilcim M, Sanders DR. The effect of combinations of flurbiprofen, low molecular weight heparin, and doxycycline on the inhibition of corneal neovascularization. Cornea 2006;25(5):582-585.
8 Yoon SY, Kim JY, Kim ES, Kim SY, Kim MJ, Tchah H. Subconjunctival injection of low-molecular-weight heparin-taurocholate 7 inhibits corneal neovascularization. Cornea 2013;32(11):1488-1492.
9 Moon CH, Moon BG, Kim JY, Kim MJ, Tchah H. Comparison of topical low-molecular-weight heparin-taurocholate and bevacizumab for treatment and prevention of corneal neovascularization. Cornea 2017;36(4):497-501.
10 Kim JY, Kim SY, Cheon MH, Kim ES, Song IS, Kim MJ, Tchah H.Attenuation of corneal neovascularization by topical low-molecularweight heparin-taurocholate 7 without bleeding complication. Int J Ophthalmol 2016;9(9):1255-1259.
11 Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol 2001;19(4):316-317.
12 Zhang X, Bloch S, Akers W, Achilefu S. Near-infrared molecular probes for in vivo imaging. Curr Protoc Cytom 2012;Chapter 12:Unit12.27.
13 Lee E, Kim YS, Bae SM, Kim SK, Jin S, Chung SW, Lee M,Moon HT, Jeon OC, Park RW, Kim IS, Byun Y, Kim SY. Polyprolinetype helical-structured low-molecular weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int J Cancer 2009;124(12):2755-2765.
14 Zou P, Xu S, Povoski SP, Wang A, Johnson MA, Martin EW Jr,Subramaniam V, Xu R, Sun D. Near-infrared fluorescence labeled anti-TAG-72 monoclonal antibodies for tumor imaging in colorectal cancer xenograft mice. Mol Pharm 2009;6(2):428-440.
15 Peterson NC, Wilson GG, Huang Q, Dimasi N, Sachsenmeier KF. Biodistribution analyses of a near-infrared, fluorescently labeled,bispecific monoclonal antibody using optical imaging. Comp Med 2016;66(2):90-99.
16 Hillman EM, Amoozegar CB, Wang T, McCaslin AF, Bouchard MB,Mansfield J, Levenson RM. In vivo optical imaging and dynamic contrast methods for biomedical research. Philos Trans A Math Phys Eng Sci 2011;369(1955):4620-4643.
17 Chopra A. Near-infrared Dye®800CW-conjugated disulfide-based cyclic RGD peptide c(CRGDKGPDC) and its DOTA analog. Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda(MD): National Center for Biotechnology Information (US); 2004-2013.2012 Aug 06 [updated 2012 Aug 30].
18 Patterson AP, Booth SA, Saba R. The emerging use of in vivo optical imaging in the study of neurodegenerative diseases. Biomed Res Int 2014;2014:401306.
19 Hu H, Huang P, Weiss OJ, Yan X, Yue X, Zhang MG, Tang Y, Nie L,Ma Y, Niu G, Wu K, Chen X. PET and NIR optical imaging using selfilluminating (64)Cu-doped chelator-free gold nanoclusters. Biomaterials 2014;35(37):9868-9876.
20 Sordillo LA, Pu Y, Pratavieira S, Budansky Y, Alfano RR. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J Biomed Opt 2014;19(5):056004.
21 Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407(6801):249-257.
22 Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol 2012;57(5):415-429.
Citation: Moon CH, Lee JY, Kim ES, Park JH, Kim SY, Kim JY,Tchah H. In vivo biodistribution of topical low molecular weight heparin-taurocholate in a neovascularized mouse cornea. Int J Ophthalmol 2018;11(9):1435-1439
Received: 2017-02-26 Accepted: 2018-07-05
DOl: 10.18240/ijo.2018.09.01
Correspondence to: Hungwon Tchah. Department of Ophthalmology, Asan Medical Center, University of Ulsan College of Medicine, #88 Olympic-ro 43-gil, Songpa-gu,Seoul 05505, Republic of Korea. hwtchah@amc.seoul.kr


