
Figure 1 Dexamethasone intravitreal implant Ozurdex implantation.
I rvine-Gass syndrome (IGS) commonly known as pseudophakic cystoid macular edema (CME) which is one of the leading causes of low visual acuity after complicated or uncomplicated cataract surgery[1-4]. Although the etiology of IGS is multifactorial, it is suggested that the main cause is the increased inflammatory mediators in aqueous and vitreous which causes disruption of the blood-aqueous and bloodretinal barriers after surgery[5-7].
Incidence of CME is higher in complicated cases related to posterior capsule rupture, iris irritation, vitreous loss, vitreous traction through the wound, vitrectomy for residual lens materials, early postoperative capsulotomy, anterior chamber intraocular lens (IOL), irisfixated IOLs, IOL dislocations and traumatic cataract. The other risk factors for CME are diabetes,uveitis, glaucoma medications and intracamaral ophthalmic solutions[8-12]. CME may occur in weeks to years after surgery but commonly it occurs in 6 to 8wk after the surgery[3-7].
Increased vascular permeability and gathered eosinophilic transudates in the outer plexiform and inner nuclear layers of the retina induces cystic cavity which combines to create larger cavity of fluid[5-7]. Although spontenous resolution of the CME is seen in most of the patients, long standing macular edema is a risk for persistant poor visual acuity in 2% of the patients[3].Rapid recognation and treatment of the syndrome is needed because of developement of subretinal fluid, lamellar hole and photoreseptor loss due to persistant macula edema[7].
Treatment of the CME was experienced with topical nonsteroidal anti-inflammatuar agents (NSAIDs), COX inhibitor (valdecoxib), oral carbonic anhydrase inhibitors(CAIs), systemic, topical, periocular, intravitreal corticosteroids as triamcinolone, anti-VEGF agents and intravitreal in fl iximab,subcutanous interferon α2a, hyperbaric theraphy, and vitrectomy[13-19]. NSAIDs inhibit the cyclooxygenase enzymes responsible for prostaglandin production[20]. Topical nepafenac(TN; Nevanac, Alcon, Puurs, Belgium) spread into the cornea and sclera and is converted to its active metabolite, amfenac,in the retina, choroid and ciliary body[21-22]. Nepafenac and amfenac block the inflammation related breakdown of blood-retina barrier[23]. Ozurdex (Allergan, Irvine, CA) is a biodegradable intravitreal drug delivery system that maintains continous delivery of the preservative free dexamethasone[24].It is demonstrated that the dexamethasone implant is effective in macular edema related with retinal vein occlusion, uveitis,diabetic macular edema, resistant macular edema and IGS[24-25].The aim of this study was to compare the safety and efficacy of TN and IVD in previously untreated IGS patients in clinical practice.
Ethical Approval The study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
This retrospective, single center study included consecutive IGS patients after phacoemulsi fication with posterior chamber IOL implantation between January 2013 and November 2015.A full ophthalmological examination, including a detailed medical history, best-corrected visual acuity (BCVA) with Early Treatment Diabetic Retinopathy Study (ETDRS) chart,slit-lamp, intraocular pressure (IOP) measurement, fundus examination and central macular thickness (CRT) measurement by spectral-domain optical coherence tomography (OCT; RS-3000 Lite, Nidek) and fundus florescein angiography (FFA)were performed in all subjects at baseline, 1, 3 and 6mo. The data comprising demographic characteristics, presence of diabetes, presence of complications associated with surgery,BCVA, IOP, CRT, fluoresce in angiography findings during the follow up period were reported. The criteria used for IGS were any of the following after cataract surgery: CRT≥250 μm;prescence of cysts on OCT; ≥30% increase in CRT from the preoperative baseline measurement; the classic petalloid leakage in the late phase on FFA[20,26].
In addition to the above criteria, the re-injection criteria were persisted macular edema at the end of 4th month of the injection and recurrence of the macular edema. History of any ocular disease as diabetic maculopathy, diabetic retinopathy,glaucoma, age related macular degeneration, uveitis, epiretinal membrane, vitreomacular traction, retinal vein or artery occlusion, any ocular surgery before cataract surgery, any other previous treatment (systemic or intravitreal) for CME,any history of systemic disease out-of control, uveitis findings in FFA were the exclusion criteria. Any of the patients did not have topical NSAIDs medication before the surgery. No selection criterion was applied for IGS treatment. Consecutive cases were alternately selected for one of two treatments:either intravitreal dexamethasone (IVD) implant injection or TN 0.1%. TN was received four times daily. The duration of IGS before treatment was 2mo for all subjects. The topical treatment group receive their treatment for 3mo. We excluded five patients from IVD group who required re-treatment with Ozurdex.

Figure 1 Dexamethasone intravitreal implant Ozurdex implantation.
Injection Technique Intravitreal injections were performed under sterile conditions in the surgery unit following standardized procedures. After instilling topical anesthetics,preoperative antisepsis was made with 5% povidone iodine.IVD was injected to the eye 3.5 mm posterior to the limbus with its 22 gauge applicator. After application the site of the injection was compressed with a cotton applicator in order to avoid vitreus re fl ux[25-26] (Figure 1).
Statistical Analysis Categorical variables were described using absolute and relative frequencies, and quantitive variables were described using mean and standard deviation. Linear mixed effects models were performed to evaluate BCVA,CMT, and IOP over the follow up period with a 95% confidence interval (CI). Mann-Whitney U test, Student's t test and Chisquare with continuity correction were used to compare the data between variables. Friedman test was used to determine the difference between the measurements. Wilcoxon signed rank test was performed for continuous variables with nonnormal distribution. The Spearman test was used to assess the correlation between variables. Statistical analysis was performed using SPSS software (version 15, SPSS Inc, IL), P value <0.05 was assumed significant for all analysis.
Totally 62 eyes of 62 IGS patients enrolled to this study. The IVD group included 32 eyes, and the TN group 30 eyes. The mean±standard deviation (SD) age of patients was 68.9±10y and 66.4±9.4y in the IVD and TN groups, respectively.Demographic data, BCVA, CRT and IOP of the two groups can be seen in Tables 1 and 2. The relation between prescence of diabetes and BCVA, CRT and IOP are shown in Table 3.The relation between prescence of complication and BCVA,CRT and IOP are shown in Table 4. Total 10 patients in IVD group and 9 patients in TN group had complications related with surgery (posterior capsule rupture, iridodialysis, vitreous incarceration, zonular dialysis).
Table 1 Demographic data of IGS patients n (%)
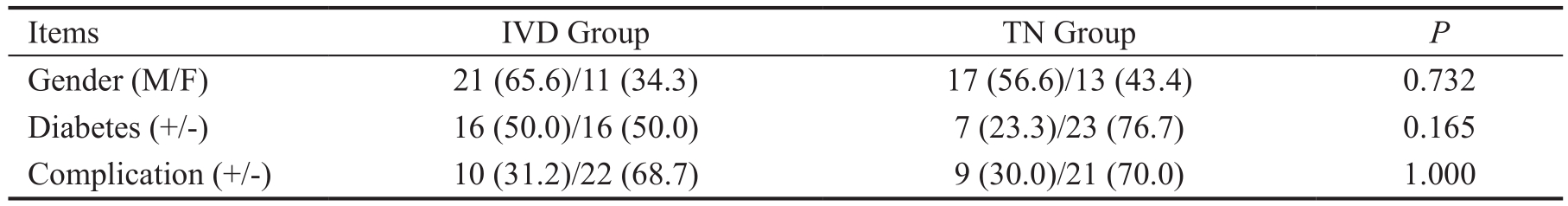
Continuity correction. IVD: Intravitreal dexamethasone; TN: Topical nepafenac.
 Gender (M/F) 21 (65.6)/11 (34.3) 17 (56.6)/13 (43.4) 0.732 Diabetes (+/-) 16 (50.0)/16 (50.0) 7 (23.3)/23 (76.7) 0.165 Complication (+/-) 10 (31.2)/22 (68.7) 9 (30.0)/21 (70.0) 1.000
Gender (M/F) 21 (65.6)/11 (34.3) 17 (56.6)/13 (43.4) 0.732 Diabetes (+/-) 16 (50.0)/16 (50.0) 7 (23.3)/23 (76.7) 0.165 Complication (+/-) 10 (31.2)/22 (68.7) 9 (30.0)/21 (70.0) 1.000
Table 2 Age, BCVA, CRT and IOP results of the IVD and TN groups
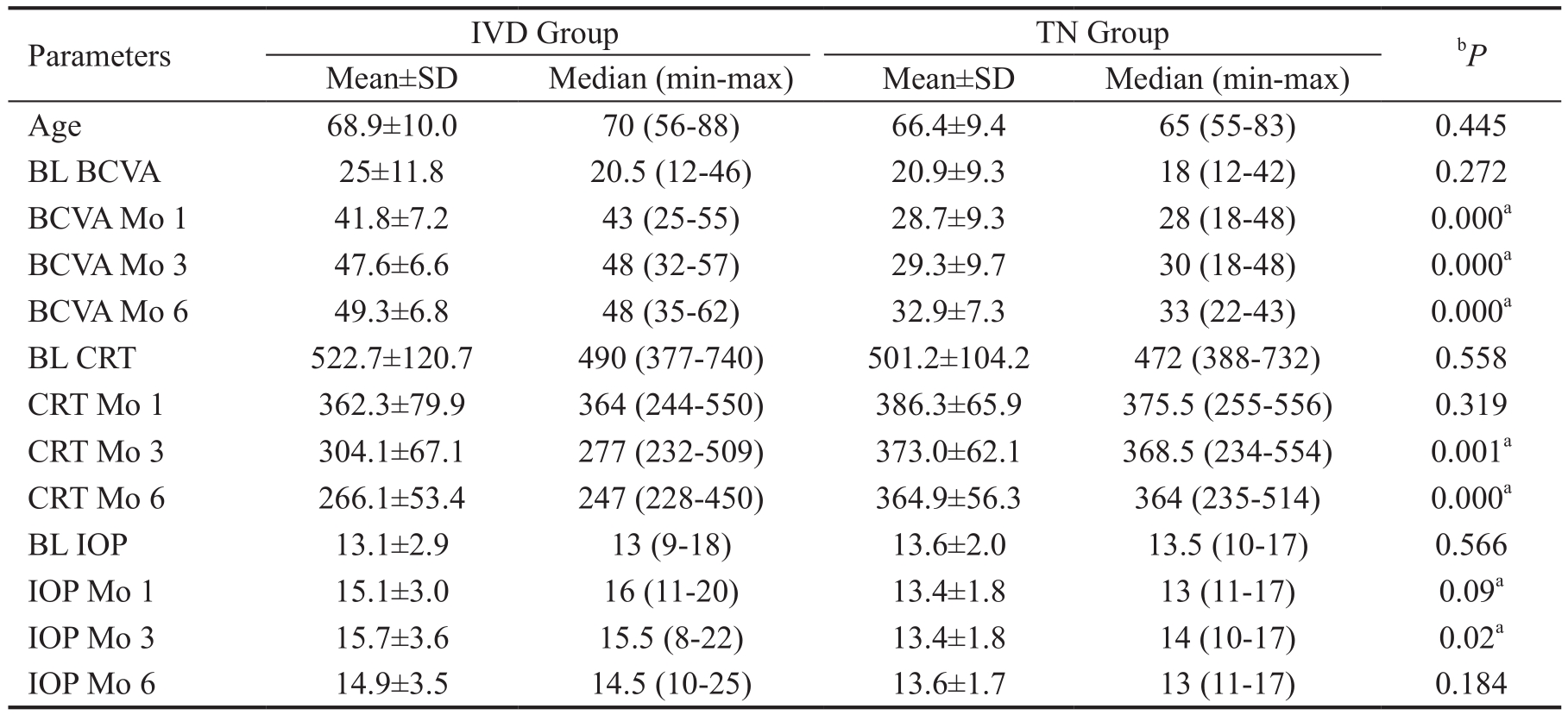
IVD: Intravitreal dexamethasone; TN: Topical nepafenac; BL: Baseline; BCVA: Best corrected visual acuity; CRT: Central retinal thickness; IOP: Intraocular pressure; Mo: Month.aStatistically significant.bMann-Whitney U test or Student's t test.
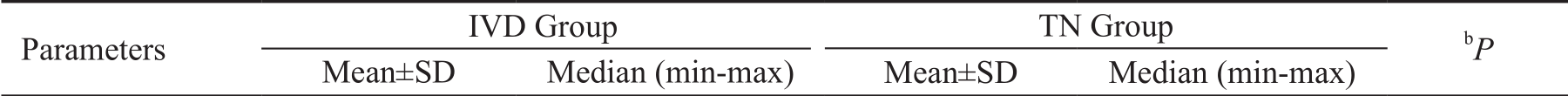 Age 68.9±10.0 70 (56-88) 66.4±9.4 65 (55-83) 0.445 BL BCVA 25±11.8 20.5 (12-46) 20.9±9.3 18 (12-42) 0.272 BCVA Mo 1 41.8±7.2 43 (25-55) 28.7±9.3 28 (18-48) 0.000a BCVA Mo 3 47.6±6.6 48 (32-57) 29.3±9.7 30 (18-48) 0.000a BCVA Mo 6 49.3±6.8 48 (35-62) 32.9±7.3 33 (22-43) 0.000a BL CRT 522.7±120.7 490 (377-740) 501.2±104.2 472 (388-732) 0.558 CRT Mo 1 362.3±79.9 364 (244-550) 386.3±65.9 375.5 (255-556) 0.319 CRT Mo 3 304.1±67.1 277 (232-509) 373.0±62.1 368.5 (234-554) 0.001a CRT Mo 6 266.1±53.4 247 (228-450) 364.9±56.3 364 (235-514) 0.000a BL IOP 13.1±2.9 13 (9-18) 13.6±2.0 13.5 (10-17) 0.566 IOP Mo 1 15.1±3.0 16 (11-20) 13.4±1.8 13 (11-17) 0.09a IOP Mo 3 15.7±3.6 15.5 (8-22) 13.4±1.8 14 (10-17) 0.02a IOP Mo 6 14.9±3.5 14.5 (10-25) 13.6±1.7 13 (11-17) 0.184
Age 68.9±10.0 70 (56-88) 66.4±9.4 65 (55-83) 0.445 BL BCVA 25±11.8 20.5 (12-46) 20.9±9.3 18 (12-42) 0.272 BCVA Mo 1 41.8±7.2 43 (25-55) 28.7±9.3 28 (18-48) 0.000a BCVA Mo 3 47.6±6.6 48 (32-57) 29.3±9.7 30 (18-48) 0.000a BCVA Mo 6 49.3±6.8 48 (35-62) 32.9±7.3 33 (22-43) 0.000a BL CRT 522.7±120.7 490 (377-740) 501.2±104.2 472 (388-732) 0.558 CRT Mo 1 362.3±79.9 364 (244-550) 386.3±65.9 375.5 (255-556) 0.319 CRT Mo 3 304.1±67.1 277 (232-509) 373.0±62.1 368.5 (234-554) 0.001a CRT Mo 6 266.1±53.4 247 (228-450) 364.9±56.3 364 (235-514) 0.000a BL IOP 13.1±2.9 13 (9-18) 13.6±2.0 13.5 (10-17) 0.566 IOP Mo 1 15.1±3.0 16 (11-20) 13.4±1.8 13 (11-17) 0.09a IOP Mo 3 15.7±3.6 15.5 (8-22) 13.4±1.8 14 (10-17) 0.02a IOP Mo 6 14.9±3.5 14.5 (10-25) 13.6±1.7 13 (11-17) 0.184
Table 3 The relation between prescence of diabetes and BCVA, CRT and IOP

IVD: Intravitreal dexamethasone; TN: Topical nepafenac; BL: Baseline; BCVA: Best corrected visual acuity; Mo: Month;CRT: Central retinal thickness; IOP: Intraocular pressure.aStatistically significant.bStudent's t test or Mann-Whitney U test.
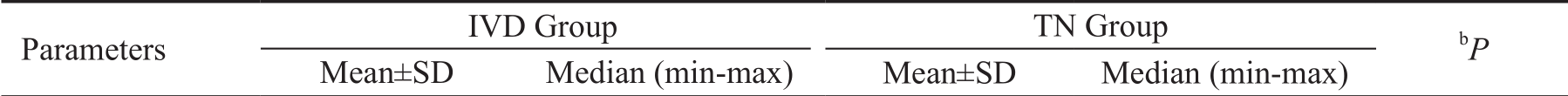 Diabetes (+)BL BCVA 27.2±9.8 24 (16-46) 18.5±8.1 16 (12-30) 0.12 BCVA Mo 1 41.9±6.0 42 (34-51) 27.0±7.8 25 (20-38) 0.85 BCVA Mo 3 47.3±6.2 48 (37-57) 30.0±9.9 27 (22-44) 0.39 BCVA Mo 6 49.2±6.3 47 (40-60) 30.5±4.2 31 (25-35) 0.94 BL CRT 555.9±124.7 612 (400-704) 500.8±82.4 479.5 (432-612) 0.48 CRT Mo 1 408±72.0 391 (314-550) 451.8±74.2 434 (383-556) 0.26 CRT Mo 3 350.5±68.4 345 (258-509) 437.5±79.1 408 (380-554) 0.02a CRT Mo 6 295.4±64.3 272 (244-450) 423.0±61.9 401.5 (375-514) 0.02a BL IOP 13.6±2.6 13 (10-18) 13.0±2.5 13.5 (10-15) 0.56 IOP Mo 1 15.7±3.0 16 (12-20) 13.8±2.8 13.5 (11-17) 0.45 IOP Mo 3 16.0±4.3 15 (8-22) 13.3±3.0 13 (10-17) 0.62 IOP Mo 6 15.9±4.3 15 (10-25) 13.0±2.2 12.5 (11-16) 0.35 Diabetes (-)BL BCVA 22.8±13.8 15 (12-44) 21.6±9.7 18 (12-42) 0.66 BCVA Mo 1 41.7±8.6 44 (25-55) 29.1±9.9 30 (18-48) 0.83 BCVA Mo 3 47.8±7.3 49 (32-57) 29.1±9.9 30 (18-48) 0.82 BCVA Mo 6 49.4±7.6 48 (35-62) 33.6±7.9 34.5 (22-49) 0.46 BL CRT 489.4±113.7 435 (377-740) 501.3±112.4 472 (388-732) 1.00 CRT Mo 1 316.1±59.9 296 (244-428) 367.6±52.1 358 (255-489) 0.04a CRT Mo 3 259.6±15.7 254 (232-280) 354.6±44.1 356.5 (234-430) 0.001a CRT Mo 6 240.6±8.5 243 (228-254) 348.4±43.9 352.5 (235-425) 0.001a BL IOP 12.7±3.3 11 (9-17) 13.8±1.9 13.5 (11-17) 0.51 IOP Mo 1 14.7±3.2 16 (11-20) 13.4±1.6 13 (11-16) 0.71 IOP Mo 3 15.4±3.0 16 (12-21) 13.5±1.5 14 (11-16) 0.74 IOP Mo 6 14.0±2.3 14 (10-17) 13.8±1.6 13.5 (11-17) 0.32
Diabetes (+)BL BCVA 27.2±9.8 24 (16-46) 18.5±8.1 16 (12-30) 0.12 BCVA Mo 1 41.9±6.0 42 (34-51) 27.0±7.8 25 (20-38) 0.85 BCVA Mo 3 47.3±6.2 48 (37-57) 30.0±9.9 27 (22-44) 0.39 BCVA Mo 6 49.2±6.3 47 (40-60) 30.5±4.2 31 (25-35) 0.94 BL CRT 555.9±124.7 612 (400-704) 500.8±82.4 479.5 (432-612) 0.48 CRT Mo 1 408±72.0 391 (314-550) 451.8±74.2 434 (383-556) 0.26 CRT Mo 3 350.5±68.4 345 (258-509) 437.5±79.1 408 (380-554) 0.02a CRT Mo 6 295.4±64.3 272 (244-450) 423.0±61.9 401.5 (375-514) 0.02a BL IOP 13.6±2.6 13 (10-18) 13.0±2.5 13.5 (10-15) 0.56 IOP Mo 1 15.7±3.0 16 (12-20) 13.8±2.8 13.5 (11-17) 0.45 IOP Mo 3 16.0±4.3 15 (8-22) 13.3±3.0 13 (10-17) 0.62 IOP Mo 6 15.9±4.3 15 (10-25) 13.0±2.2 12.5 (11-16) 0.35 Diabetes (-)BL BCVA 22.8±13.8 15 (12-44) 21.6±9.7 18 (12-42) 0.66 BCVA Mo 1 41.7±8.6 44 (25-55) 29.1±9.9 30 (18-48) 0.83 BCVA Mo 3 47.8±7.3 49 (32-57) 29.1±9.9 30 (18-48) 0.82 BCVA Mo 6 49.4±7.6 48 (35-62) 33.6±7.9 34.5 (22-49) 0.46 BL CRT 489.4±113.7 435 (377-740) 501.3±112.4 472 (388-732) 1.00 CRT Mo 1 316.1±59.9 296 (244-428) 367.6±52.1 358 (255-489) 0.04a CRT Mo 3 259.6±15.7 254 (232-280) 354.6±44.1 356.5 (234-430) 0.001a CRT Mo 6 240.6±8.5 243 (228-254) 348.4±43.9 352.5 (235-425) 0.001a BL IOP 12.7±3.3 11 (9-17) 13.8±1.9 13.5 (11-17) 0.51 IOP Mo 1 14.7±3.2 16 (11-20) 13.4±1.6 13 (11-16) 0.71 IOP Mo 3 15.4±3.0 16 (12-21) 13.5±1.5 14 (11-16) 0.74 IOP Mo 6 14.0±2.3 14 (10-17) 13.8±1.6 13.5 (11-17) 0.32
Table 4 The relation between prescence of complication during surgery and BCVA, CRT and IOP

IVD: Intravitreal dexamethasone; TN: Topical nepafenac; BL: Baseline; BCVA: Best corrected visual acuity; CRT: Central retinal thickness; IOP: Intraocular pressure; Mo: Month.aStatistically significant.bStudent's t test or Mann-Whitney U test.
 Complication (+)BL BCVA 23.67±3.6 22 (15-38) 22.0±13.4 14 (12-42) 1.00 BCVA Mo 1 38.50± 3.4 39 (25-49) 29.6±12.9 24 (18-48) 0.17 BCVA Mo 3 43.67±3.4 44 (32-56) 31.2±13.7 24 (18-48) 0.07 BCVA Mo 6 44.83±2.8 45 (35-56) 32.8±10.3 32 (23-49) 0.04a BL CRT 562±52.3 577 (412-704) 512.8±103.6 514 (388-618) 0.36 CRT Mo 1 355.1±59.5 363 (285-438) 440±73.7 425 (352-556) 0.06 CRT Mo 3 347.5±85.4 333 (275-509) 430.4±73.7 417 (352-554) 0.06 CRT Mo 6 300.3±80.3 279 (235-450) 416.66±62.7 402 (341-514) 0.04a BL IOP 12.7±2.4 11 (10-15) 14±2.3 15 (10-16) 0.34 IOP Mo 1 14.0±2.2 14 (11-16) 14.8±2.2 15 (11-17) 0.29 IOP Mo 3 15.3±4.6 15 (8-22) 14.0±2.5 14 (10-17) 0.96 IOP Mo 6 14.8±3.4 14 (10-20) 13.8±1.9 14 (11-16) 0.92 Complication (-)BL BCVA 25.67±3.8 20.5 (12-46) 20.4±7.7 18 (12-36) 0.80 BCVA Mo 1 43.42±1.7 44.5 (34-55) 28.3±8.1 30 (18-42) 0.80 BCVA Mo 3 49.50±1.3 49.0 (43-57) 28.6±8.1 30 (18-42) 0.62 BCVA Mo 6 51.58±1.6 50.5 (44-62) 33.0±6.2 34 (22-45) 0.96 BL CRT 502±33.8 457 (377-740) 496.6±108.2 456 (398-732) 0.87 CRT Mo 1 365.9±90.7 296 (244-550) 365.5±51.5 358 (255-489) 0.41 CRT Mo 3 283.9±46.6 265 (232-377) 350.9±40.8 358 (234-386) 0.001a CRT Mo 6 251.9±22.5 246 (228-312) 345.0±40.5 355 (235-379) 0.001a BL IOP 13.5±3.0 13.5 (9-18) 13.4±1.9 13 (11-17) 0.62 IOP Mo 1 15.6±3.2 16 (11-20) 12.9±1.3 13 (11-15) 0.06 IOP Mo 3 15.9±3.2 15 (12-22) 13.2±1.5 13 (11-16) 0.39 IOP Mo 6 15.0±3.6 14 (10-25) 13.5±1.6 13 (11-17) 0.65
Complication (+)BL BCVA 23.67±3.6 22 (15-38) 22.0±13.4 14 (12-42) 1.00 BCVA Mo 1 38.50± 3.4 39 (25-49) 29.6±12.9 24 (18-48) 0.17 BCVA Mo 3 43.67±3.4 44 (32-56) 31.2±13.7 24 (18-48) 0.07 BCVA Mo 6 44.83±2.8 45 (35-56) 32.8±10.3 32 (23-49) 0.04a BL CRT 562±52.3 577 (412-704) 512.8±103.6 514 (388-618) 0.36 CRT Mo 1 355.1±59.5 363 (285-438) 440±73.7 425 (352-556) 0.06 CRT Mo 3 347.5±85.4 333 (275-509) 430.4±73.7 417 (352-554) 0.06 CRT Mo 6 300.3±80.3 279 (235-450) 416.66±62.7 402 (341-514) 0.04a BL IOP 12.7±2.4 11 (10-15) 14±2.3 15 (10-16) 0.34 IOP Mo 1 14.0±2.2 14 (11-16) 14.8±2.2 15 (11-17) 0.29 IOP Mo 3 15.3±4.6 15 (8-22) 14.0±2.5 14 (10-17) 0.96 IOP Mo 6 14.8±3.4 14 (10-20) 13.8±1.9 14 (11-16) 0.92 Complication (-)BL BCVA 25.67±3.8 20.5 (12-46) 20.4±7.7 18 (12-36) 0.80 BCVA Mo 1 43.42±1.7 44.5 (34-55) 28.3±8.1 30 (18-42) 0.80 BCVA Mo 3 49.50±1.3 49.0 (43-57) 28.6±8.1 30 (18-42) 0.62 BCVA Mo 6 51.58±1.6 50.5 (44-62) 33.0±6.2 34 (22-45) 0.96 BL CRT 502±33.8 457 (377-740) 496.6±108.2 456 (398-732) 0.87 CRT Mo 1 365.9±90.7 296 (244-550) 365.5±51.5 358 (255-489) 0.41 CRT Mo 3 283.9±46.6 265 (232-377) 350.9±40.8 358 (234-386) 0.001a CRT Mo 6 251.9±22.5 246 (228-312) 345.0±40.5 355 (235-379) 0.001a BL IOP 13.5±3.0 13.5 (9-18) 13.4±1.9 13 (11-17) 0.62 IOP Mo 1 15.6±3.2 16 (11-20) 12.9±1.3 13 (11-15) 0.06 IOP Mo 3 15.9±3.2 15 (12-22) 13.2±1.5 13 (11-16) 0.39 IOP Mo 6 15.0±3.6 14 (10-25) 13.5±1.6 13 (11-17) 0.65
There was a statistically significant difference in the post treatment BCVA values both in the IVD group and in the TN group depending on the time (P=0.000, P=0.000 respectively;Friedman test). In IVD group there was a statistically significant difference between baseline BCVA and BCVA post treatment month 1, BCVA post treatment 1-3mo, BCVA post treatment 1-6mo and, BCVA post treatment 3-6mo (P=0.000,0.000, 0.000, 0.005, respectively; Wilcoxon signed ranks test).In TN group there was a statistically significant difference between baseline BCVA and BCVA post treatment 1mo,BCVA post treatment 1-6mo and BCVA post treatment 3-6mo(P=0.000, 0.004, 0.008, respectively; Wilcoxon signed ranks test).
In IVD group there was not a statistically significant difference between baseline CRT and CRT post treatment 1mo (P=1.00,Wilcoxon signed ranks test), however in TN group there was a statistically significant difference between baseline CRT and CRT post treatment 1mo (P=0.0001; Wilcoxon signed ranks test). We found a statistically significant difference between post treatment CRT 1-3mo (in IVD group P=0.0001, in TN group P=0.002; Wilcoxon signed ranks test), and post treatment CRT 3-6mo in both groups (in IVD group P=0.002, in TN group P=0.0001, Wilcoxon signed ranks test; Figures 2 and 3).
In IVD group there was a statistically significant difference between the baseline IOP and IOP post treatment month 1 (P=0,001, Wilcoxon signed ranks test). We found no difference between the IOP post treatment 1-3mo (in IVD group P=0.312, in TN group P=1.0; Wilcoxon signed ranks test) and post treatment 3-6mo (in IVD group P=0.376, in TN group P=0.544, Wilcoxon signed ranks test) in both groups.There was a statistically significant difference in the post treatment CRT values both in the IVD group and in the TN group depending on the time (P=0.000, P=0.000 respectively,Friedman test). There was a statistically significant difference in the post treatment IOP values in IVD group depending on time however there was no significant difference in the post treatment IOP values in TN group (P=0.000, 0.701 respectively; Friedman test; Figures 4-6).

Figure 2 Dexamethasone implant treatment
A: Baseline macular OCT image of a patient in IVD group; B: After 1 dexamethasone implant in 6mo.
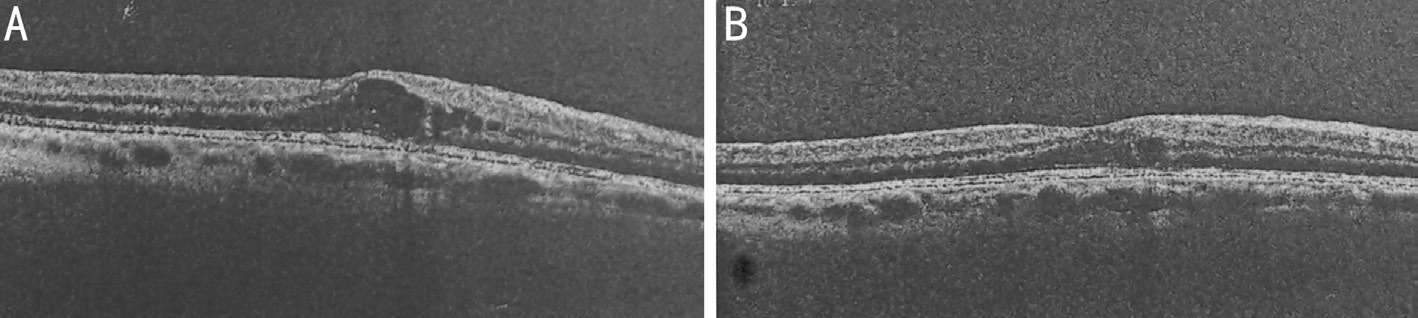
Figure 3 Topical Nepafenac treatment
A: Baseline macular OCT image of a patient in TN group; B: OCT image of the patient after 6mo with nepafenac treatment.
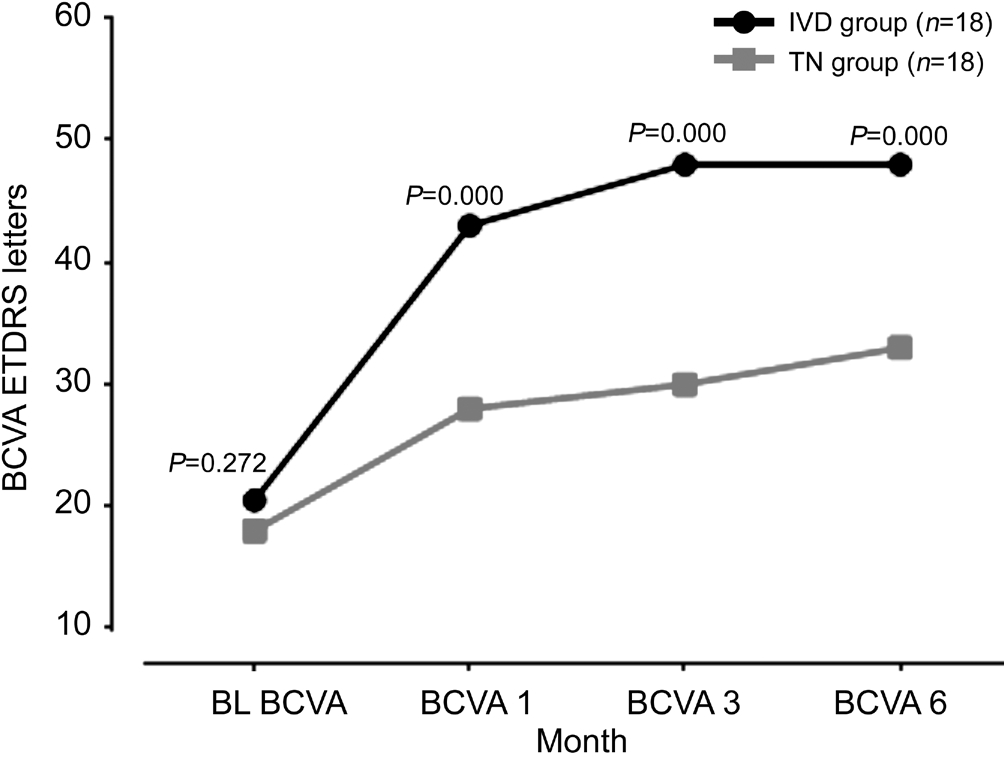
Figure 4 Changes in mean BCVA and P value of IVD and TN groups during each visit.

Figure 5 Changes in mean CRT and P value of IVD and TN groups during the follow-up period.
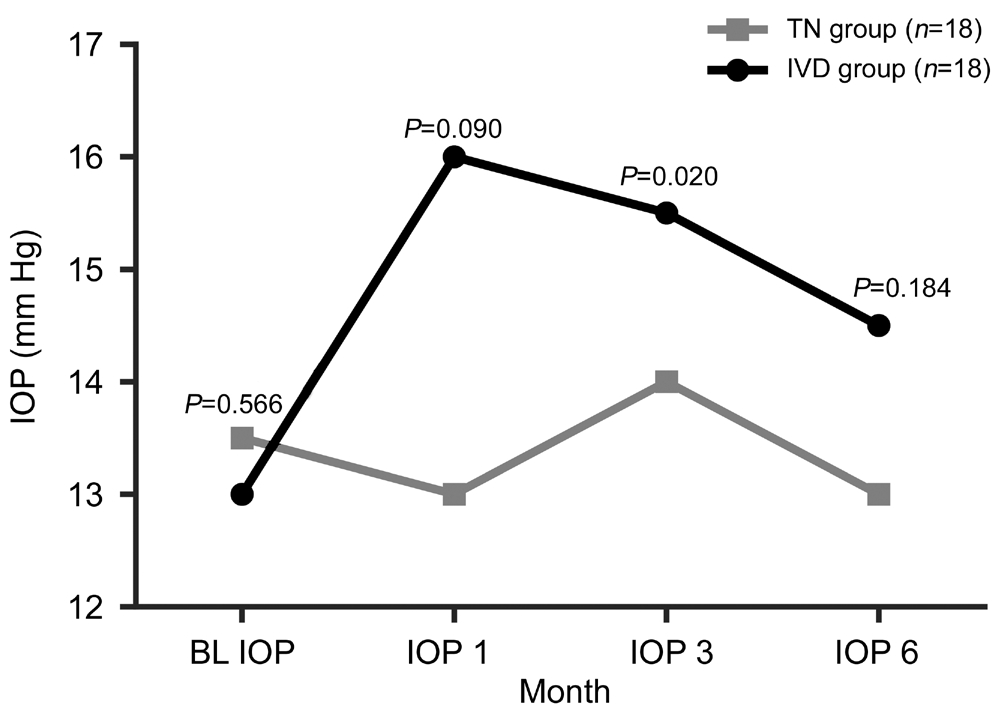
Figure 6 Changes in mean IOP and P value of IVD and TN groups during the follow-up period.
There was no correlation between age and the post-treatment BCVA month 6 and post-treatment CRT month 6 in IVD(r=0296, P=0.23, r=-0.171, P=0.49 respectively) and TN group(r=-0.02, P=0.93, r=0.27, P=0.27 respectively; Spearman's test). Baseline BCVA has a positive correlation with the post treatment BCVA month 6 in TN group (r=0.75, P=0.001;Spearman's test). There was no correlation between baseline BCVA and post treatment BCVA month 6 in IVD group(r=0.33, P=0.17; Spearman's test). There was no correlation between baseline CRT and post treatment CRT month 6 in both IVD and TN group (Tables 5 and 6).
Three patients with increased IOP (≥7 mm Hg increase from average baseline IOP) were managed successfully with standard topical medications; none required surgery. Five of the patients required ≤3 injection. Three of the patients had persistant CME and required re-injection, two of the patients had recurrent CME. Six patients had mild to moderate superficial punctate keratitis in TN group. No other systemic or local complication occured in IVD and TN group after the treatments.
In the present study, nepafenac ophthalmic solution 0.1%and dexamethasone intravitreal implant was used in treating pseudophakic cystoid macula edema. The dexamethasone group had higher visual acuity in all post treatment visits.
Table 5 Correlation analysis of age, baseline BCVA, final BCVA at month 6 and IOP in IVD group
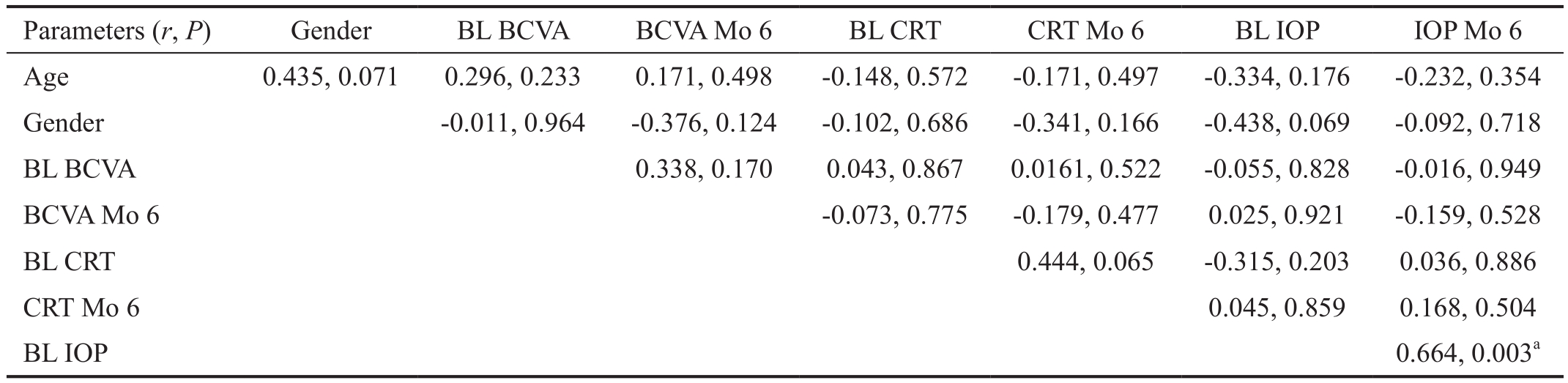
BL: Baseline; BCVA: Best corrected visual acuity; Mo: Month; CRT: Central retinal thickness; IOP: Intraocular pressure. Spearman's correlation.aStatistically significant.
 Age 0.435, 0.071 0.296, 0.233 0.171, 0.498 -0.148, 0.572 -0.171, 0.497 -0.334, 0.176 -0.232, 0.354 Gender -0.011, 0.964 -0.376, 0.124 -0.102, 0.686 -0.341, 0.166 -0.438, 0.069 -0.092, 0.718 BL BCVA 0.338, 0.170 0.043, 0.867 0.0161, 0.522 -0.055, 0.828 -0.016, 0.949 BCVA Mo 6 -0.073, 0.775 -0.179, 0.477 0.025, 0.921 -0.159, 0.528 BL CRT 0.444, 0.065 -0.315, 0.203 0.036, 0.886 CRT Mo 6 0.045, 0.859 0.168, 0.504 BL IOP 0.664, 0.003a
Age 0.435, 0.071 0.296, 0.233 0.171, 0.498 -0.148, 0.572 -0.171, 0.497 -0.334, 0.176 -0.232, 0.354 Gender -0.011, 0.964 -0.376, 0.124 -0.102, 0.686 -0.341, 0.166 -0.438, 0.069 -0.092, 0.718 BL BCVA 0.338, 0.170 0.043, 0.867 0.0161, 0.522 -0.055, 0.828 -0.016, 0.949 BCVA Mo 6 -0.073, 0.775 -0.179, 0.477 0.025, 0.921 -0.159, 0.528 BL CRT 0.444, 0.065 -0.315, 0.203 0.036, 0.886 CRT Mo 6 0.045, 0.859 0.168, 0.504 BL IOP 0.664, 0.003a
Table 6 Correlation analysis of age, baseline BCVA, final BCVA at month 6 and IOP in TN group
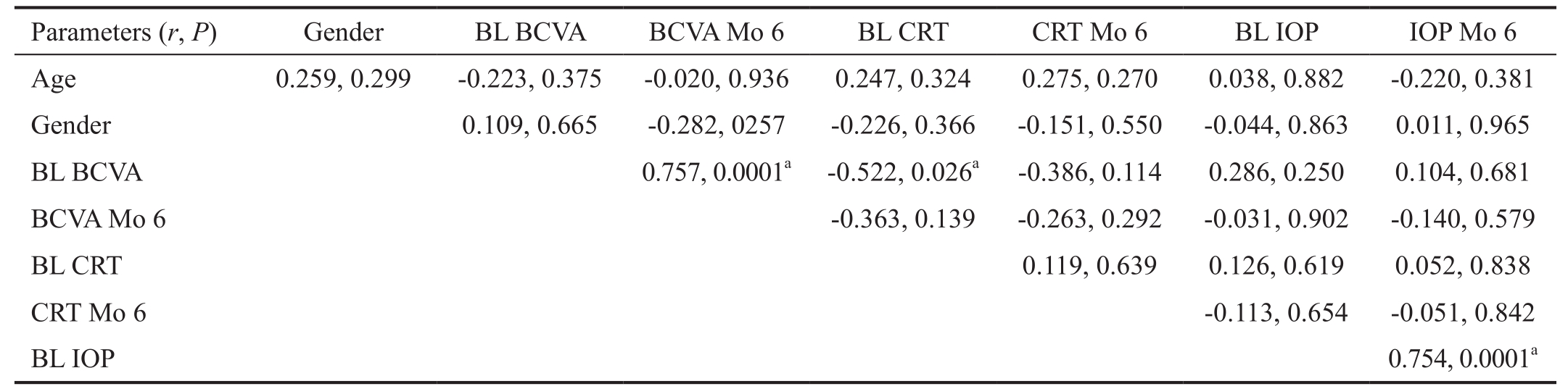
Spearman's correlation. BL: Baseline; BCVA: Best corrected visual acuity; Mo: Month, CRT: Central retinal thickness; IOP: Intraocular pressure.aStatistically significant.
?
IVD group also had significantly lower CRT in post treatment month 3 and month 6. However, IVD group had no significant different IOP rates than TN group in post treatment month 3 and month 6.
IGS was fifirst reported in 1953 as CME after cataract extraction[1,10]. Description of the disease supported with angiographic findings by Gass and Norton. Clinical findings of IGS (poor visual acuity and metamorphopsia) is seen in 0.1%-2% patients and it is detectable via OCT in 4%-11% patients after modern cataract surgery[27-32]. Although spontenous healing of the CME seen in IGS, it could be resistant and lead to irreversible injury to the macula and cause poor visual acuity in some of the patients[3,7,10].
Although IGS usually described as postsurgical CME,Bellocq et al[18] considered that IGS (macular edema after phacoemulsification surgery) and other postsurgical macular edema (vitrectomy for retinal detachment or epiretinal membrane peeling) could be two different entities. Because they reported a significant functional and anatomical improvement in IGS poor prognosis in other postsurgical macular edema have been associated with underlying macular disease[18]. Therefore in the present study we included the patients who have only phacoemulsification surgery to eliminate the macular and vitreomacular interface diseases.
The pathogenesis of IGS was reported to be multifactorial but inlammation is suggested as the major cause of IGS[4,10]. The releasing of multiple factors (histamin, prostaglandins and seratonin, bradykinin, acetylcholine, small peptides) induce Inflammation and cause breakdown of the blood-retinal barrier and lead to macular edema[10,13,33]. Altough the major underlying cause is well known, there is no consensus on standart treatment protocol in IGS. The most common treatment is oral acetazolamide and topical NSAIDs combination[10]. Systemic acetazolamide had multiple adverse effects such as cramps,renal colic, asthenia and tingling. Multiple studies reported that topical NSAIDs speed the recovery of blood-aqueous barrier and decrease Inflammation after cataract surgery[13,34-37].
Nepafenac is acyclooxygenase inhibitor. It has been shown to have 6 times faster corneal permeability than diclofenac[38].Animal studies and clinical studies emphasized that topical NSAIDs such as nepafenac and bromfenac had increased penetration to the posterior segment[39-42]. Kapin et al[22]demonstrated that TN passes through the posterior segment and it decreases vitreous protein and PGE2 concentrations.In a recent article[23], none of the other topical NSAIDs have inhibited inflammation to the same degree. Such higher penetration to the posterior segment provides utilization of TN in IGS. Warren et al[43] and Ghanbari et al[44] suggested that TN and bromfenac could be more effective in combination with intravitreal corticosteroids and anti-VEGFs for chronic pseudophakic CME. A recently published prospective study showing the superiority of nepafenac comparison with subtenon steroids in controlling pseudophakic CME[28]. In contrast, the present study showed that IVD was more effective than TN in previously untreated IGS.
A number of studies have reported conflicting outcomes of using bevacizumab in IGS. Barone et al[3] reported functional and anatomical improvement in a limited patient group. Spitzer et al[45] reported that postoperative pseudophakic CME will not benefit from intravitreal bevacizumab.
Ranibizumab is the other anti-VEGF treatment option for IGS. Case series have suggested its safety and efficacy for the treatment of pseudophakic CME[46-47]. A flibercept was found to be effective traetment in a case report but the patient required multiple injections for cure[48].
The positive effects of intravitreal triamcinolone were demonstrated, however repeated intravitreal injections were required[49-50]. Chin et al[51] reported that triamcinolone acetonide reduces faster in vitrectomized eyes after injection. However, it is suggested in multiple studies that dexamethasone implant is effective in vitrectomized eyes[5,52].It is suggested that the patients with pre-existing glaucoma and steroid response are more likely to have severe increased IOP after intravitreal triamcinolone acetonide[53]. On the other hand recent study demonstrated that IVD implant has an admissible safety profile even in eyes with glaucoma and the eyes which are receiving treatment for ocular hypertension[54].Even if increased IOP occured, it could be well controlled by a strict monitorization and topical treatment[55-56]. In the present study only three patient received topical treatment for IOP≤25 mm Hg and none of the patients required filtering surgery. As mentioned in multiple studies this result may be related with the sustained release mechanism of the implant which provides more controlled drug delivery to cause lower side effects[52,57]. Infliximab which is an anti tumor necrosis factor (TNF) α agent had positive effect in IGS but side effects such as retinal toxicity is reported[15,56].
In a recent study, TN has a significant narrowing effect on retinal arteriolar diameter in eyes with nonproliferative diabetic retinopathy[58]. Studies using retinal vessel caliber showed narrowing after a single-intravitreal triamcinolone acetonide application[59-60]. The injection of ranibizumab and bevacizumab also had constrictive effects on retinal blood vessel diameter[61]. The effect of nepafenac and its product,amfenac, involves retinal angiogenesis, inhibiting the effects of VEGF by reducing vasodilatation[62]. TN might have constrictor effects on the retinal artery trunk and may decrease CRT in eyes with diabetic macula edema[58].
In the current study, topical treatment group receive their treatment for 3mo. All the patients were compliant in using the TN treatment. Pollack et al[63] reported a 90d nepafenac treatment prevented macular edema after cataract surgery in patients with diabetic retinopathy and they demonstrated no safety issues within the study group.
Dexamethasone implant is the second step treatment for IGS.EPISODIC-2 study reported anatomical and functional positive effects in IGS. Bellocq et al[16] reported the predictive factors of functional effectiveness and they reported the patients who had at least one post surgical macular edema risk factor as capsular rupture, uveitis, retinal vein occlusion, diabetes, epiretinal membrane are more tend to develop IGS. Age, history of systemic or topical treatments, time to the initial injection were not predictive factors. Initial visual acuity was demonstrated as a predictive factor in EPISODIC-2 study[16]. In this study baseline visual acuity was correlated with final visual acuity in TN group, however there was no significant correlation between baseline visual acuity and final visual acuity at 6mo in IVD group. This outcome shows that the dexamethasone implant is superior to the long term nepafenac treatment in any case. Age was not correlated with final BCVA and CRT results in both group.
Mayer et al[10] reported in their prospective nonrandomized study that BCVA increased from 30.2±4.3 letters to 50.4±4.9 letters at 12mo with IVD in IGS. In EPISODIC-2 study naive status was demonstrated as a predictive factor related with lower risk of recurrence[16]. We included the patients who had naive status which means the patients had no treatment(systemic or topical) before for IGS. In our study the patients baseline BCVA was not different in IVD and TN groups.Visual acuity was improved at the end of the 6mo in both groups. CRT is determining the anatomical effectiveness[16].Mayer et al[10] showed decreased foveal thickness from 520.8 µm to 232.8 µm at month 12 and in their study, 9 patients had recurrence after 3mo and needed re-treatment with IVD. Multiple previous studies have reported no adverse events related to IVD except the increased IOP which was controlled with only medical treatment[63-65]. In the present study we also found similar results with the literature in IVD group at post-treatment month 6 in terms of BCVA, CRT and adverse events.
Limitations of the present study are lack of control group and relatively short follow-up period. However to the best of our knowledge this study is the fifirst study that comparing IVD injection with TN for treatment of IGS.
In conclusion, both TN and IVD found to be safe and effective in reducing macula edema and increasing visual acuity in previously untreated IGS patients. However according to the 6-month study results, IVD has been shown to be more effective method than TN in terms of clinical outcomes with very low complication rates. Currently, there is no standart treatment modality for IGS. We believe that our study will help to indicate a standart treatment protocol for IGS. However,further prospective large sample size clinical studies are needed to reveal the best treatment algorithm in IGS.
Conflicts of Interest: Guclu H, None; Pelitli Gurlu V, None.
1 Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol 1953;36(5):599-619.
2 Drolsum L, Haaskjold E. Causes of decreased visual acuity after cataract extraction. J Cataract Refract Surg 1995;21(1):59-63.
3 Barone A, Russo V, Prascina F, Delle Noci N. Short-term safety and efficacy of intravitreal bevacizumab for pseudophakic cystoid macular edema. Retina 2009;29(1):33-37.
4 Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol 2012;23(1):26-32.
5 Dutra Medeiros M, Navarro R, Garcia-Arumí J, Mateo C, Corcóstegui B. Dexamethasone intravitreal implant for treatment of patients with recalcitrant macular edema resulting from irvine-gass syndrome. Invest Ophthalmol Vis Sci 2013;54(5):3320.
6 Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin 2010;50(1):139-153.
7 Reis A, Birnbaum F, Hansen LL, Reinhard T. Successful treatment of cystoid macular edema with valdecoxib. J Cataract Refract Surg 2007;33(4):682-685.
8 Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 1998;96:557-634.
9 Cohen SM, Davis A, Cukrowski C. Cystoid macular edema after pars plana vitrectomy for retained lens fragments. J Cataract Refract Surg 2006;32(9):1521-1526.
10 Mayer WJ, Kurz S, Wolf A, Kook D, Kreutzer T, Kampik A, Priglinger S, Haritoglou C. Dexamethasone implant as an effective treatment option for macular edema due to Irvine-Gass syndrome. J Cataract Refract Surg 2015;41(9):1954-1961.
11 Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysis. Ophthalmology 1998;105(3):397-405.
12 Cicik ME, Doğan C, Arslan OŞ. Effect of intracameral ophthalmic cefuroxime solution (Aprokam®) in the prophylaxis of cataract surgery in patients with keratoplasty. Balkan Med J 2018;35(2):181-185.
13 Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina 2011;31(1):4-12.
14 Sivaprasad S, Bunce C, Wormald R. Non-steroidal anti-inflammatory agents for cystoid macular oedema following cataract surgery: a systematic review. Br J Ophthalmol 2005;89(11):1420-1422.
15 Wu L, Arevalo JF, Hernandez-Bogantes E, Roca JA. Intravitreal infliximab for refractory pseudophakic cystoid macular edema: results of the Pan-American Collaborative Retina Study Group. Int Ophthalmol 2012;32(3):235-243.
16 Bellocq D, Pierre-Kahn V, Matonti F, et al. Effectiveness and safety of dexamethasone implants for postsurgical macular oedema including Irvine-Gass syndrome: the EPISODIC-2 study. Br J Ophthalmol 2017;101(3):333-341.
17 Garcia JM, Isaac DL, Ávila MP. Dexamethasone 0.7 mg implants in the management of pseudophakic cystoid macular edema. Arq Bras Oftalmol 2016;79(2):113-115.
18 Bellocq D, Korobelnik JF, Burillon C, Voirin N, Dot C, Souied E,Conrath J, Milazzo S, Massin P, Baillif S, Kodjikian L. Effectiveness and safety of dexamethasone implants for post-surgical macular oedema including Irvine-Gass syndrome: the EPISODIC study. Br J Ophthalmol 2015;99(7):979-983.
19 Arevalo JF, Maia M, Garcia-Amaris RA, Roca JA, Sanchez JG,Berrocal MH, Wu L, Pan-American Collaborative Retina Study Group.Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results.Ophthalmology 2009;116(8):1481-1487,1487.e1.
20 Singh RP, Lehmann R, Martel J, Jong K, Pollack A, Tsorbatzoglou A,Staurenghi G, Cervantes-Coste Cervantes G, Alpern L, Modi S, Svoboda L, Adewale A, Jaffe GJ. Nepafenac 0.3% after cataract surgery in patients with diabetic retinopathy: results of 2 randomized phase 3 studies.Ophthalmology 2017;124(6):776-785.
21 Ke TL, Graff G, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of traumainduced ocular Inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 2000;24(4):371-384.
22 Kapin MA, Yanni JM, Brady MT, McDonough TJ, Flanagan JG, Rawji MH, Dahlin DC, Sanders ME, Gamache DA. Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation 2003;27(5):281-291.
23 Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM.Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular Inflammation: I. Assessment of antiinflammatory efficacy. Inflammation 2000;24(4):357-370.
24 London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther 2011;28(5):351-366.
25 Mylonas G, Georgopoulos M, Malamos P, Georgalas I, Koutsandrea C, Brouzas D, Sacu S, Perisanidis C, Schmidt-Erfurth U, Macula Study Group Vienna. Comparison of dexamethasone intravitreal implant with conventional triamcinolone in patients with postoperative cystoid macular edema. Curr Eye Res 2017;42(4):648-652.
26 Ayar O, Alpay A, Koban Y, Akdemir MO, Yazgan S, Canturk Ugurbas S,Ugurbas SH. The effect of dexamethasone intravitreal implant on retinal nerve fiber layer in patients diagnosed with branch retinal vein occlusion.Curr Eye Res 2017;42(9):1287-1292.
27 Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. 1966. Retina 2003;23(6 Suppl):646-661.
28 Yüksel B, Uzunel UD, Kerci SG, Sağban L, Küsbeci T, Örsel T.Comparison of subtenon triamcinolone acetonide injection with topical nepafenac for the treatment of pseudophakic cystoid macular edema. Ocul Immunol Inflamm 2017;25(4):513-519.
29 Schmier JK, Halpern MT, Covert DW, Matthews GP. Evaluation of costs for cystoid macular edema among patients after cataract surgery.Retina 2007;27(5):621-628.
30 Wittpenn JR, Silvefirstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M, Acular LS for Cystoid Macular Edema (ACME) Study Group. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol 2008;146(4):554-560.
31 Henderson BA, Kim JY, Ament CS, Ferru fino-Ponce ZK, Grabowska A,Cremers SL. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg 2007;33(9):1550-1558.
32 Bélair ML, Kim SJ, Thorne JE, Dunn JP, Kedhar SR, Brown DM,Jabs DA. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am J Ophthalmol 2009;148(1):128-135.e2.
33 Pande MV, Spalton DJ, Kerr-Muir MG, Marshall J. Postoperative inflammatory response to phacoemulsi fication and extracapsular cataract surgery: aqueous fl are and cells. J Cataract Refract Surg 1996;22(Suppl 1):770-774.
34 Diestelhofirst M, Aspacher F, Konen W, Krieglstein GK. The effect of flurbiprofen 0.03% eye drops on the blood aqueous barrier in extracapsular cataract extraction with IOL implantation. Int Ophthalmol 1991;15(2):69-73.
35 Kraff MC, Sanders DR, McGuigan L, Raanan MG. Inhibition of bloodaqueous humor barrier breakdown with diclofenac. A fl uorophotometric study. Arch Ophthalmol 1990;108(3):380-383.
36 Flach AJ. Cyclo-oxygenase inhibitors in ophthalmology. Surv Ophthalmol 1992;36(4):259-284.
37 Flach AJ. Topical nonsteroidal antiinflammatory drugs in ophthalmology. Int Ophthalmol Clin 2002;42(1):1-11.
38 Lane SS, Modi SS, Lehmann RP, Holland EJ. Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular Inflammation associated with cataract surgery. J Cataract Refract Surg 2007;33(1):53-58.
39 Seth A, Ghosh B, Raina UK, Gupta A, Arora S. Intravitreal diclofenac in the treatment of macular edema due to branch retinal vein occlusion.Ophthalmic Surg Lasers Imaging Retina 2016;47(2):149-155.
40 Soheilian M, Karimi S, Ramezani A, Montahai T, Yaseri M, Soheilian R, Peyman GA. Intravitreal diclofenac versus intravitreal bevacizumab in naive diabetic macular edema: a randomized double-masked clinical trial.Int Ophthalmol 2015;35(3):421-428.
41 Soheilian M, Eskandari A, Ramezani A, Rabbanikhah Z, Soheilian R.A pilot study of intravitreal diclofenac versus intravitreal triamcinolone for uveitic cystoid macular edema. Ocul Immunol Inflamm 2013;21(2): 124-129.
42 Tsilimbaris MK, Tsika C, Kymionis GD. Intravitreal ketorolac for the treatment of chronic cystoid macular edema after cataract surgery. Ther Clin Risk Manag 2016;12:177-182.
43 Warren KA, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina 2010;30(2):260-266.
44 Ghanbari H, Kianersi F, Sonbolestan SA, Abtahi MA, Akbari M,Abtahi ZA, Abtahi SH. Intravitreal Diclofenac plus Bevacizumab versus Bevacizumab alone in treatment-naive diabetic macular edema: a randomized double-blind clinical trial. Int Ophthalmol 2017;37(4):867-874.
45 Spitzer MS, Ziemssen F, Yoeruek E, Petermeier K, Aisenbrey S,Szurman P. efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J Cataract Refract Surg 2008;34(1):70-75.
46 Mitropoulos PG, Chatziralli IP, Peponis VG, Drakos E, Parikakis EA.Intravitreal ranibizumab for the treatment of irvine-gass syndrome. Ocul Immunol Inflamm 2015;23(3):225-231.
47 Demirel S, Batioğlu F, Özmert E. Intravitreal ranibizumab for the treatment of cystoid macular edema in irvine-gass syndrome. Journal of Ocular Pharmacology and Therapeutics 2012;28(6):636-639.
48 Lin CJ, Tsai YY. Use of a flibercept for the management of refractory pseudophakic macular edema in irvine-gass syndrome and literature review. Retin Cases Brief Rep 2018;12(1):59-62.
49 Conway MD, Canakis C, Livir-Rallatos C, Peyman GA. Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular edema. J Cataract Refract Surg 2003;29(1):27-33.
50 Konstantopoulos A, Williams CP, Luff AJ. Outcome of intravitreal triamcinolone acetonide in postoperative cystoid macular oedema. Eye(Lond) 2008;22(2):219-222.
51 Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005;25(5):556-560.
52 Chang-Lin JE, Burke JA, Peng Q, Lin T, Orilla WC, Ghosn CR,Zhang KM, Kuppermann BD, Robinson MR, Whitcup SM, Welty DF.Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci 2011;52(7):4605-4609.
53 Jain S, Thompson JR, Foot B, Tatham A, Eke T. Severe intraocular pressure rise following intravitreal triamcinolone: a national survey to estimate incidence and describe case profiles. Eye (Lond) 2014;28(4):399-401.
54 Theodoropoulou S, Ellabban AA, Johnston RL, Cilliers H, Mohamed Q, Sallam AB. Short-term safety of dexamethasone implant for treatment of macular edema due to retinal vein occlusion, in eyes with glaucoma or treated ocular hypertension. Graefes Arch Clin Exp Ophthalmol 2017;255(4):725-732.
55 Malclès A, Dot C, Voirin N, Vié AL, Agard É, Bellocq D, Denis P,Kodjikian L. Safety of intravitreal dexamethasone implant (ozurdex):the SAFODEX study. incidence and risk factors of ocular hypertension.Retina 2017;37(7):1352-1359.
56 Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, Falk N. Adverse events after intravitreal infliximab (Remicade). Retina 2010;30(1):71-80.
57 Furino C, Boscia F, Recchimurzo N, Sborgia C, Alessio G. Intravitreal dexamethasone implant for refractory macular edema secondary to vitrectomy for macular pucker. Retina 2014;34(8):1612-1616.
58 Evliyaoğlu F, Akpolat Ç, Kurt MM, Çekiç O, Nuri Elçioğlu M. Retinal vascular caliber changes after topical nepafenac treatment for diabetic macular edema. Cur Eye Res 2018;43(3):357-361.
59 Kurt MM, Çekiç O, Akpolat Ç, Aslankurt M, Elçioğlu M. Vessel diameter study: intravitreal vs posterior subtenon triamcinolone acetonide injection for diabetic macular edema. Eye 2017;31(8):1155-1162.
60 Wickremasinghe SS, Rogers SL, Gillies MC, Zhu MD, Wong TY.Retinal vascular caliber changes after intravitreal triamcinolone treatment for diabetic macular edema. Invest Ophthalmol Vis Sci 2008;49(11):4707-4711.
61 Kurt MM, Çekiç O, Akpolat Ç, Elçioglu M. Effects of intravitreal ranibizumab and bevacizumab on the retinal vessel size in diabetic macular edema. Retina 2018;38(6):1120-1126.
62 Yanni SE, Clark ML, Yang R, Bingaman DP, Penn JS. The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res Bull 2010;81(2-3):310-319.
63 Pollack A, Staurenghi G, Sager D, Mukesh B, Reiser H, Singh RP.Prospective randomised clinical trial to evaluate the safety and efficacy of nepafenac 0.1% treatment for the prevention of macular oedema associated with cataract surgery in patients with diabetic retinopathy. Br J Ophthalmol 2017;101(4):423-427.
64 Sudhalkar A, Chhablani J, Vasavada A, Bhojwani D, Vasavada V,Vasavada S, Medscape. Intravitreal dexamethasone implant for recurrent cystoid macular edema due to Irvine-Gass syndrome: a prospective case series. Eye (Lond) 2016;30(12):1549-1557.
65 Sze AM, Luk FO, Yip TP, Lee GK, Chan CK. Use of intravitreal dexamethasone implant in patients with cataract and macular edema undergoing phacoemulsi fication. Eur J Ophthalmol 2015;25(2):168-172.