
·Basic Research·
Changes
in corneal innervation and pain responses in fungal keratitis
Chang-Jie
Ren, Yi-Fan Zhou, Yuan Wu, Xu-Dong Peng, Cui Li, Qian Wang, Guo-Qiang Zhu, Jia
You, Jie Zhang, Gui-Qiu Zhao, Jing Lin
Department
of Ophthalmology, the Affiliated Hospital of Qingdao University, Qingdao 26600,
Shandong Province, China
Co-first authors: Chang-Jie Ren and Yi-Fan Zhou
Correspondence
to: Jing Lin and
Gui-Qiu Zhao. Department of Ophthalmology, the Affiliated Hospital of Qingdao
University, No. 16 Jiangsu Road, Qingdao 26600, Shandong Province, China.
yankelinjing@126.com
Received:
Abstract
AIM: To
characterize changes in the cornea nerve and pain responses in fungal keratitis
(FK).
METHODS:
A retrospective analysis of in vivo confocal microscopy images of 11 FK
corneas was performed, and the results were compared with those for 11 normal
corneas. Subbasal corneal nerves were analyzed for total nerve number, main
nerve trunk number, branching patterns and tortuosity. C57BL/6 mice were
infected with Aspergillus fumigatus. Disease severity was determined
through clinical scoring and slit lamp photography. Corneas were harvested at
1, 3, 5, and 7d post infection (p.i.) and assessed for β III tubulin. Corneal mechanical sensitivity thresholds
were detected by von Frey test. β-endorphin (β-EP) and μ receptor protein expression was detected through Western
blotting.
RESULTS:
Total nerve number, main nerve trunk number, and nerve branching were
significantly lower in FK patients than in controls, but tortuosity was not
significantly different. In infected mice, subbasal nerve density decreased
from 1d p.i., reaching a minimum at 5d p.i. Clinical scores rose at 1d p.i.,
peaked at 3d p.i., and decreased at 5d p.i. Mechanical sensitivity thresholds
showed the same trends. β-EP and μ receptor protein expression increased after infection.
CONCLUSION:
Corneal nerve density is lower in FK patients and Aspergillus fumigatus-infected
mice than in controls. Pain sensitivity decreases with postinfection corneal
ulcer aggravation. β-EP and μ receptor proteins are both upregulated in infected mouse
corneas.
KEYWORDS:
keratitis; pain; fungal; innervation; subbasal nerve; mice
DOI:10.18240/ijo.2020.01.01
Citation: Ren
CJ, Zhou YF, Wu Y, Peng XD, Li C, Wang Q, Zhu GQ, You J, Zhang J, Zhao GQ, Lin
J. Changes in corneal innervation and pain responses in fungal keratitis. Int
J Ophthalmol 2020;13(1):1-6
INTRODUCTION
Fungal keratitis (FK) is a popular
eye disease worldwide, especially in developing countries dominated by
agricultural populations[1]. Recently, the
incidence of this disease has elevated dramatically[2].
In the clinic, FK patients often present with decreased corneal pain response,
which causes patients to lose opportunities for early treatment and causes
substantial vision damage that produces a need for corneal transplantation. The
lack of pain responses highlights the complex mechanisms underlying FK symptoms
and the difficulty in alleviating them. The reason for pain insensitivity in FK
has not been clarified; however, such clarification is important not only to
enable timely and effective treatment of FK patients but also to further
understand the pathogenic mechanisms of FK.
The cornea is the most densely
innervated structure in the human body[3]. Corneal
nerves not only play essential roles in eye sensations of pain, touch and
temperature but also have effect on blink reflexs, wound healing, and tear
secretion[4]. Sensory nerve endings of the corneal
surface are defined as nociceptors. Eye pain is mediated by nociceptors of the
trigeminal nerve terminals located on corneas[5].
In addition to the conduction of pain, peripheral nerves also contain
endogenous analgesic protein receptors. β-endorphin (β-EP) is an endogenous
opioid peptide in peripheral tissue that can play an important role in pain
control[6-7]. β-EP has strong
affinity for both µ and δ receptors, and it mainly acts on μ receptors to
produce its effects. Opioid receptors produce antinociceptive or analgesic
effects[8]. The number of peripheral opioid
receptors has been reported to increase in the inflammatory state[9]. In acute inflammation, opioid peptides are almost
exclusively expressed by immune cells[10-11].
After the opioid peptide is released, it acts on the corresponding receptors of
peripheral sensory nerve endings to trigger antinociception[12].
This mechanism has been proven in inflammation of surrounding tissues such as
the skin[13-14], but the
expression of these molecules has not yet been studied in FK.
It is important to elucidate the
reason underlying the lack of pain response in FK. In this study, we focused on
changes in corneal nerves and pain responses in FK. We show that corneal nerve
density was lower after Aspergillus fumigatus (A. fumigatus)
infection. Pain sensitivity decreased with postinfection corneal ulcer
aggravation. β-EP and μ receptor proteins were both upregulated in infected
mouse corneas.
MATERIALS AND METHODS
Ethical Approval All patients were informed of the
purpose of the study and their consent was obtained in accordance with the
Declaration of Helsinki. All mice were treated in a humane way according to the
Association for Research in Vision and Ophthalmology (ARVO) Statement for the
Use of Animals in Ophthalmic and Visual Research.
In Vivo Confocal Microscopy A retrospective review of patients
with FK in the Affiliated Hospital of Qingdao University between January 2013
and November 2018 was carried out. By obtaining positive culture results from
microbiological laboratory data, and searching the confocal microscope results
of Confoscan 4 slit-scanning confocal microscope (Nidek Co. Ltd., Japan), found
cases of filamentous hyphae positive in the cases. Exclusion criteria for FK
patients included wearing contact lens, past history of infectious keratitis,
ocular inflammatory disease or eye trauma; ophthalmic surgery for the first
three months; or diabetes. Examination of all patients was performed by the
same ophthalmologist. The affected eye was anesthetized using 0.5% proparacaine
eye drops, and the head and eyes were fixed in front of the microscope. The
examiner applied an appropriate amount of gel to the lens and adjusted the
handle on the main unit to bring the gel on the lens into contact with the
cornea. Images were saved for data analysis. For each subject, three
high-quality subbasal nerve bundle images were selected for analysis. For image
analysis, we referred to the criteria and methods used by Kurbanyan et al[15]. Image J was used to analyzed and calculated all
parameters retrospectively by a blinded observer.
Animals and Corneal Infection Jinan Pengyue Experimental Animal
Co. Ltd. (Jinan, China) provided C57BL/6 mice (female, 8wk) without specific
pathogens. The corneas were examined distinctly with slit lamp microscope
before use in experiments. To anesthetize mice, 8% chloral hydrate (0.08
mL/mouse) was applied via intraperitoneal injection. Mice corneas were
scraped to form a wound (
Immunohistochemistry After cervical dislocation of the
mice, mouse eyeballs were removed and fixed with 1.3% paraformaldehyde in
phosphate buffer saline (PBS) at room temperature for 1h. Then, the corneas
were dissected, and radial incisions were made to ensure that the corneal
tissues could be flat-mounted. The corneas were then washed in PBS five times
for five minutes per wash, then 1% Triton X
Von Frey Test To examine the pain response after
infection, a behavioral test was performed. The von Frey test was employed to
examine corneal mechanical sensitivity thresholds[17].
The mice were wrapped in surgical towels beneath a stereoscopic microscope and
gently held by hand to ensure that the eye was completely exposed. A set of
calibrated von Frey hairs (Stoelting Co., IL, USA) was used to probe the areas
surrounding the ulcer of the cornea. Blink response was assessed in untreated
controls and mice infected with A. fumigatus (n=5 per group); a
positive response for the test was recorded when a mouse exhibited a blink
response. Each cornea was mechanically stimulated five times with the von Frey
hairs (0.008, 0.02, 0.04, 0.07, 0.16 and
Western Blot Analysis Proteins were isolated from corneas
using RIPA buffer (Solarbio, Beijing, China) mixed with phenylmethanesulfonyl
fluoride (PMSF) (100:1, Solarbio) for 2h. The concentration of total proteins
was evaluated with BCA Protein Assay Reagent (Solarbio). Western blotting
system was established and performed as previously described[2].
Primary antibodies against pomc (1:3000; Elabscience, Wuhan, China), µ receptor
protein (1:1000; Abcam, Cambridge, UK) and GAPDH (1:3000; R&D, Minneapolis,
MN) were applied.
Statistics Statistical analyses of the
differences between the two groups were performed using two-tailed Student’s t-tests
in GraphPad Prism 5.0. The relationships between the clinical scores and the
von Frey hair forces were assessed by Spearman rank correlation analysis. All
data are expressed as the mean±standard deviation (SD). P<0.05 was
considered statistically significant.
RESULTS
In Vivo Confocal Microscopy Analysis in
Fungal Keratitis Patients Diagnoses of all patients were
confirmed by finding out fungal hyphae on in vivo confocal
microscopy (IVCM) or by positive culture results in microbiology laboratory
analysis. Among the 11 FK patients, 6 were infected with Fusarium and 5
were infected with Aspergillus, and the patients had suffered from
corneal ulcers for 12±4d. The clinical results are summarized in Table 1.
Table 1 Demographic data of patients
with fungal keratitis in comparison with normal controls mean±SD
|
Parameters |
No. of patients (n) |
Age (y) |
Gender (M/F) |
Days of infection (d) |
|
Normal |
11 |
40±16 |
6/5 |
- |
|
Fungal |
11 |
37±10 |
5/6 |
12±4 |
Days of infection denotes the time
elapsed since diagnosis of the infection until the IVCM or fungal scraping was
performed.
The discovered nerves were noted to
have quantitative and morphological changes in FK patients (Figure
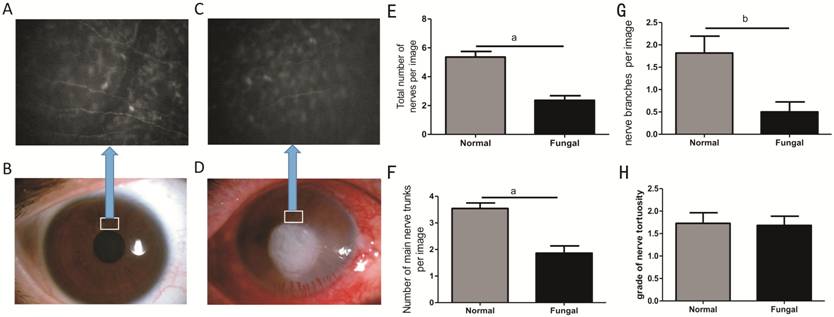
Figure 1 Representative the slit-lamp
photographs and in vivo confocal microscopy (IVCM) images of normal
cornea and FK patients IVCM
analysis showing a reduced total nerve count in FK patients (C) compared with
normal corneas (A). D and B are the slit-lamp photographs of FK patient and
normal cornea respectively. The total nerve counts were significantly lower in
patients with FK than in normal controls (E). The average number of main nerve
trunks was also significantly lower in the FK group than in the normal control
group (F). Nerve branching was found to be diminished in FK corneas compared to
normal corneas (G). However, tortuosity was not significantly different between
the FK group and the normal group (H). aP<0.001, bP<0.01.
Clinical Scores and Von Frey Test
Results in C57BL/6 Mice To illustrate the disease response
in infected mice, we used a slit lamp to take photographs at 1, 3, 5, and 7d
post infection (p.i.; Figure
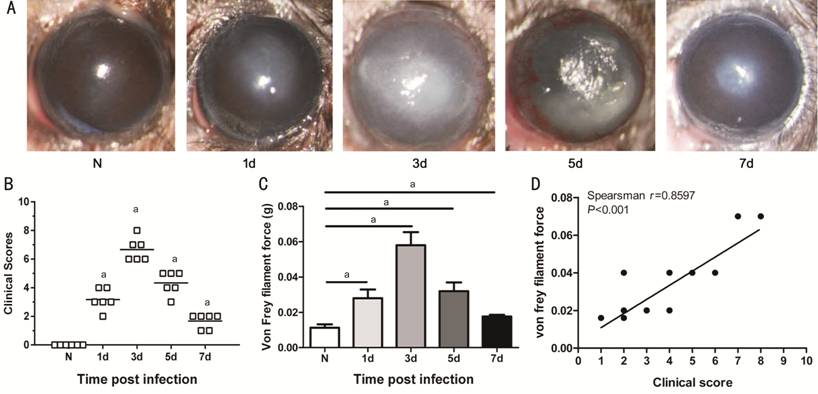
Figure 2 Clinical score and Von Frey
test in C57BL/6 mice Take photographs at 1, 3, 5,
and 7d p.i. to illustrate the disease response in infected mice (A). The
clinical score (B) increased from 1d p.i., peaked at 3d p.i., declined by 5d
p.i. and was reduced to the lowest level at 7d p.i. The von Frey filament force
(C) was increased at 1d p.i. (P<0.01) and peaked at 3d p.i. There was
a strong positive correlation between clinical scores and force of Von Frey
filament (D). aP<0.001.
Changes in Corneal Nerves in
Infected C57BL/6 Mice To research the effect of A.
fumigatus infection on corneal nerve structure and to examine the
relationship between structure and pain insensitivity, C57BL/6 mice corneas
were harvested to take immunohistochemical (IHC) staining with an β III tubulin
antibody, which against the panneuronal marker, at 1, 3, 5, and 7d p.i. Under
the microscope, we found that the nerve structure could not be clearly observed
in the central ulcer area of the cornea after A. fumigatus infection,
possibly due to severe corneal edema caused by the infection. In addition, the
nerve could not be stained normally. We thus observed and imaged the areas
surrounding the ulcer. As we can see in the corneal whole-mount images (Figure
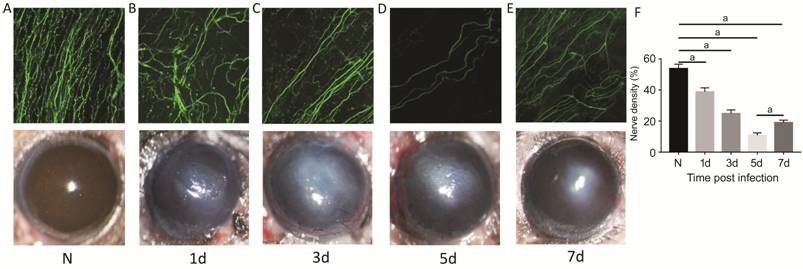
Figure 3 Changes of corneal nerves
in infected C57BL/6 mice In comparison to the normal
group (A), progressive nerve loss starting at 1d p.i. (B), with more pronounced
loss at 3d p.i. (C) and 5d p.i. (D). The number of nerves at 7d p.i. (E), was
higher than that at 5d p.i. Magnification (A-E): 200×. Quantification of
fluorescence integrated density of β III tubulin using Image J (F). aP<0.001.
Expression of β-EP and the μ
Receptor in Infected C57BL/6 Mice To further study the causes of pain
insensitivity in FK, we tested the protein levels of β-EP and the μ receptor in
normal (uninfected) and infected C57BL/6 corneas by Western blot analysis. The
results indicated that β-EP protein levels (Figure
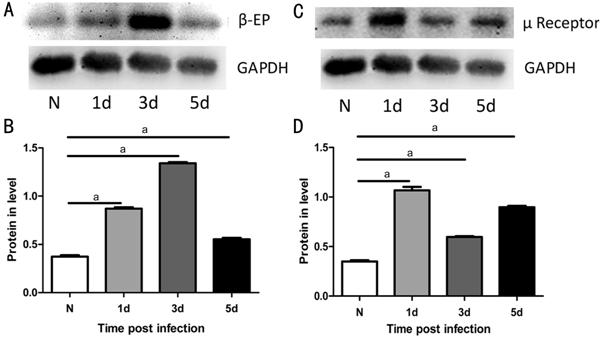
Figure 4 Expression of β-EP and μ
receptor in infected C57BL/6 mice β-EP protein
levels (A, B) were upregulated in infected mouse corneas compared with normal
corneas at 1, 3 and 5d p.i., peaked at 3d p.i. At the same time, μ receptor
protein (C, D) was also upregulated at 1, 3 and 5d p.i. in the infected group
compared with the normal group. aP<0.001.
DISCUSSION
FK is a severe infective corneal
disease caused by pathogenic fungi that is accompanied by high rates of
blindness[18]. However, in the clinic, treatment
is delayed in many FK patients due to a lack of severe pain, resulting in
corneal perforation and even loss of vision. In the current study, FK patients
who had been infected for 12±4d presented with mild pain, confirming the lack
of pain responses in FK. (It would be better if the Cochet-Bonnet esthesiometry
test was used to evaluate corneal sensation in patients, but since our research
is retrospective and esthesiometry is not our routine examination, we did not
perform it.)
The cornea is mainly dominated by
sensitive fibers which originated from the eye area of the trigeminal ganglion[3]. Eye pain is caused by nociceptors expressed on the
nerve endings of the trigeminal neurons that innervate the surface of the
eye. Chucair-Elliott et al[19] found that the loss of corneal sensation in herpes
stromal keratitis is related to decreases in corneal sensory nerves. In our
study, we found that the total number of nerves, average number of main nerve
trunks and degree of nerve branching were all lower in FK patients than in
normal subjects. These findings are consistent with previous studies on the
corneal nerves of FK patients[15].
To further explore the change in
corneal nerves and pain responses, we established an A. fumigatus mouse
model. For the first time, we found that the number of subbasal corneal nerves
was decreased in the infection group, with the lowest level at 5d p.i. To
evaluate topical resiniferatoxin (RTX) as a corneal analgesic, Bates et al[17] performed von Frey tests to analyze mechanical
sensitivity thresholds in corneas. To evaluate pain responses after A.
fumigatus infection, we similarly performed von Frey tests at various time
points after infection. In our research, the clinical score was highest at 3d
p.i., but the force of corneal touch needed to elicit a blinking response was
also the greatest at this time point. Moreover, we also found that corneas
became less sensitive to pain with increasing severity of ulceration. The
results showed that there was a significant correlation between the clinical
score and the von Frey hair force needed to elicit a response. The results also
indicated that with increasing clinical scores, corneal subbasal nerve density
decreased and the von Frey hair force needed to elicit a blink response increased.
We noticed that corneas had the least sensitivity at 3d p.i.; however, the
number of subbasal nerves at 5d p.i. was fewer than 3d p.i. We speculate that
the recovery of pain at 5d p.i. may is due to the reduction of inflammatory
cells, causing the release of analgesic substances to decrease.
There were endogenous analgesic
protein receptors in the peripheral nerves. β-EP is a major member of the
endogenous analgesic system. When inflammation occurs, inflammatory cells
release β-EP, which then acts on receptors on the peripheral nerves to
inactivate ion channels and hyperpolarize the nerves, thereby exerting an
analgesic effect[20-21]. To
explore whether β-EP and its μ receptor are expressed in A. fumigatus
keratitis, we detected their protein levels for the first time. The results
showed that both β-EP and μ receptor protein levels were significantly elevated
by A. fumigatus infection. β-EP protein expression was upregulated in
infected mouse corneas compared with normal corneas from 1d p.i. and peaked at
3d p.i. At the same time, μ receptor protein expression was also upregulated at
1d p.i., 3d p.i. and 5d p.i. compared with the normal group. A previous study
reported that P-enkephalin (P-ENK, a precursor of the endogenous opioid
enkephalin) mRNA levels were significantly decreased in patients with dry eyes
who had significant pain[22]. In addition,
another study[23] demonstrated that treatment
with an anti-β-EP antibody reduced endogenous antinociceptive activity in rats
infected with unilateral hindpaw inflammation induced by Freund’s adjuvant. Our
findings are consistent with studies showing that the numbers of opioid
receptors in the periphery are increased during inflammation[9].
In summary, the data presented here
indicated that both FK patients and C57BL/6 mice infected with A. fumigatus
had profound reductions in subbasal corneal nerves compared with normal
controls. In infected mouse models, increased ulcer severity was associated
with reduced pain sensitivity. Furthermore, β-EP and its μ receptor were both
upregulated after infection. Extensive studies are needed to investigate the
specific mechanisms by which nerve degeneration and endogenous analgesic
protein activity mediate pain insensitivity in FK.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural
Science Foundation of China (No.81470609; No.81870632); the Youth National
Natural Science Foundation of China (No.81700800; No.81800800; No.81500695);
Natural Science Foundation of Shandong Province (No.ZR2017MH008; No.ZR2017BH025).
Conflicts of Interest: Ren CJ, None; Zhou YF,
None; Wu Y, None; Peng XD, None; Li C, None; Wang Q,
None; Zhu GQ, None; You J, None; Zhang J, None; Zhao
GQ, None; Lin J, None.
REFERENCES