
·Clinical
Research·
Different
modes of foveal regeneration after closure of full-thickness macular holes by
(re)vitrectomy and autologous platelet concentrate
Andreas
Bringmann, Claudia Jochmann, Jan Darius
Unterlauft, Renate Wiedemann, Matus Rehak, Peter
Wiedemann
Department of Ophthalmology and Eye
Hospital, University of Leipzig, Leipzig 04103, Germany
Correspondence
to: Peter
Wiedemann. Department of Ophthalmology and Eye Hospital, University of Leipzig,
Liebigstrasse 10-14, Leipzig 04103, Germany.
Peter.Wiedemann@medizin.uni-leipzig.de
Received:
Abstract
AIM: To describe using
spectral-domain optical coherence tomography the regeneration of the foveal
morphology after pars plana (re)vitrectomy surgery and gas tamponade combined
with injection of autologous platelet concentrate to treat full-thickness
macular holes, and to describe different anatomical outcome.
METHODS: A retrospective case series
of 8 eyes of 8 patients was described.
RESULTS: In all cases
investigated, the platelet-assisted closure of macular holes was associated
with a rapid resolution of cystic cavities in the foveal walls. In two
patients, there was a regular regeneration of the foveal morphology after hole
closure; the regenerated central fovea had a regular structure with a foveola
and photoreceptors. In three other patients, there was an irregular
regeneration of the fovea; a foveola was not formed, photoreceptor cells were
absent from the foveal center, and the center was composed of Müller and
retinal pigment epithelial (RPE) cells. The foveal regeneration after hole
closure may proceed with or without a temporary detachment of the foveal center
from the RPE, and with or without a direct contact between the central outer
nuclear layer (ONL) and the RPE. Contacts between the ONL and RPE were observed
only in patients with an irregular foveal regeneration after hole closure.
CONCLUSION: The data show that
there are different modes of foveal regeneration after closure of macular holes
with (re)vitrectomy and platelet concentrate. It is suggested that the regular
regeneration of the foveal morphology proceeds by Müller cell-mediated tissue
movements without cell proliferation, whereas the irregular foveal regeneration
proceeds in part by proliferation of Müller and RPE cells.
KEYWORDS: macular hole; platelet
concentrate; fovea; Müller glia; retinal pigment epithelium
DOI:10.18240/ijo.2020.01.06
Citation:
Bringmann A, Jochmann C, Unterlauft JD, Wiedemann R, Rehak M, Wiedemann P.
Different modes of foveal regeneration after closure of full-thickness macular
holes by (re)vitrectomy and autologous platelet concentrate. Int J
Ophthalmol 2020;13(1):36-48
INTRODUCTION
The fovea as the site of the
sharpest vision is a pit in the inner retina over an area of elongated thin
photoreceptors. The fovea consists of the foveola which is surrounded by foveal
walls. The inner retinal layers are absent from the foveola and radially
shifted to the parafovea; this configuration needs special structural support.
Mechanical stabilization of the retina is normally provided by macroglia,
especially Müller cells[1]. The structure of the
fovea is stabilized by Müller glia, the only type of macroglia in the fovea[2-3]. The central fovea does not contain
blood vessels; a vascular ring in the foveal slope delimitates the foveal
avascular zone[4].
The fovea contains two populations
of Müller cells: foveolar Müller cells which constitute the so-called Müller
cell cone, and Müller cells of the foveal walls; because the outer processes of
the Müller cells of the foveal walls traverse horizontally or obliquely the
Henle fiber layer (HFL), they have a Z-shape[2,5-6]. The horizontal layer of the Müller
cell cone constitutes the innermost layer of the foveola; the vertical stalk of
the Müller cell cone is localized in the center of the foveola[3,7]. The Müller cell cone increases the
resistance against mechanical stress resulting from anteroposterior and
tangential tractions[2-3,7].
Disruption of the Müller cell cone produces a macular hole[2].
On the other hand, tractions exerted by foveal Müller cells are also suggested
to be involved in the regeneration of the foveal morphology after spontaneous
or surgical closure of macular holes[8-9].
The mechanisms which mediate the
closure of macular holes are largely unclear. Because astrocytes are absent
from the fovea[3], the closure is likely mediated
by Müller cells. Small macular holes may close spontaneously with a low rate,
between 0 and 6% in various studies[10-11].
It was suggested that the closure of small holes proceeds by proliferation of
glial cells which bridge the gap in the central fovea[10].
On the other hand, the finding that the volume of the foveal tissue remains
unaltered after surgical closure of macular holes[12]
suggests that glial proliferation is less relevant. It was also suggested that
the hole closure proceeds without glial proliferation[13]
by Müller cell-mediated tissue movements[8-9].
The finding that only small holes with diameters of less than 400 µm may close
spontaneously[10-11] was
explained with the assumption that the range of the Müller cell-mediated tissue
displacement is defined by the diameter of the foveola[9].
The current surgical management of
macular holes includes pars plana vitrectomy, excision of the posterior
vitreous cortex with or without internal limiting membrane (ILM) peeling,
intraocular gas tamponade, and postoperative facedown position[14]. Various adjuvant therapies to increase the surgical
success rate were described. One of the most effective adjuvant is
administration of autologous platelet concentrate onto the macular hole[15-18]. The mechanisms of the positive
effect of platelets on the closure of macular holes are unclear. There is
evidence that this effect is mediated by growth factors released from the
cells, but independent on the cellular adhesion capability, i.e., the
sealing action of aggregated platelets[19].
Platelets release numerous growth factors[20]
which accelerate tissue repair[21] and stimulate
the proliferation and migration of retinal glial and retinal pigment epithelial
(RPE) cells[22-26]. In the
present study, we describe using spectral-domain optical coherence tomography
(SD-OCT) the anatomical regeneration of the fovea in 8 eyes of 8 patients which
underwent (re)vitrectomy with autologous platelet concentrate to close
persistent macular holes.
SUBJECTS AND METHODS
Ethical Approval This retrospective study followed
the ethical standards of the Declaration of Helsinki. Eight eyes of 8 patients
with idiopathic macular hole, referred to the Department of Ophthalmology,
Medical Faculty of the University of Leipzig, Germany, between February 2009
and March 2019, were included in the study. Informed consent was obtained from
all patients.
The patients underwent a 20-, 23-,
or 25-gauge three-port pars plana (re)vitrectomy. If applicable, the ILM was
peeled after staining with Brilliant Blue G (0.125 mg;
Seven patients were of Caucasian
descent and one of African descent. All patients had central visual loss and
metamorphopsia in one or both eyes. With the exception of one case, the foveas
in the fellow eyes had a normal morphology with or without vitreomacular
adhesions. Patient 1 was a 63 year-old man who presented a full-thickness
macular hole in the right eye (Figure
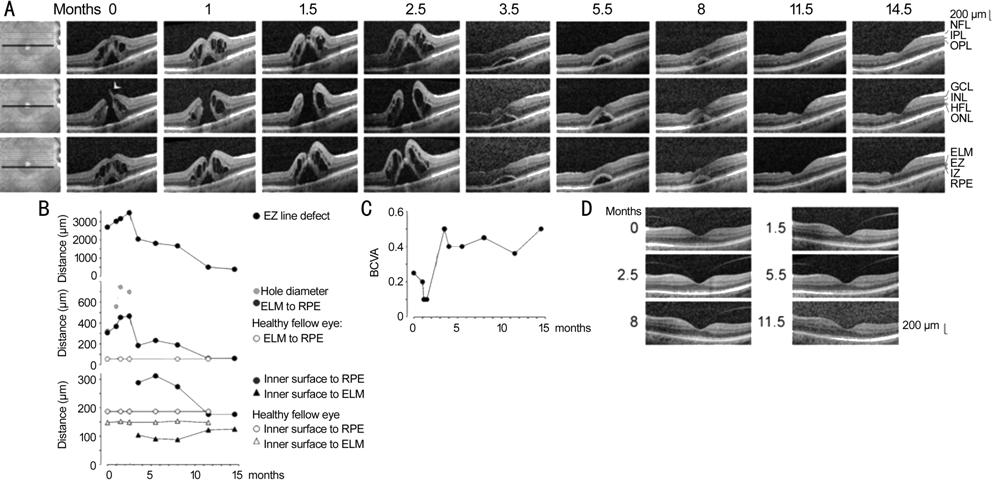
Figure 1 Closure of a macular hole after
revitrectomy with autologous platelet concentrate in the right eye of patient
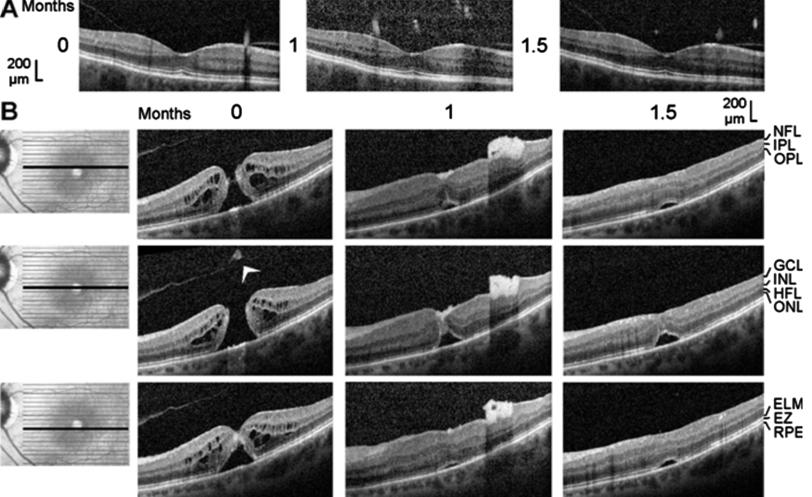
Figure 2 Closure of a macular hole after
vitrectomy with autologous platelet concentrate in the left eye of patient 2 The optical coherence tomographical
images were recorded during the first visit (0mo) and 1 and 1.5mo later. A:
Linear horizontal scans through the fovea of the right eye. The fovea showed no
structural abnormalities with the exceptions of the vitreomacular adhesions at the
parafoveas and the thickened hyperreflectivity in the inner layer of the
foveola. B: Linear horizontal scans through the fovea and parafovea of the left
eye. The orientation of the scans are shown at the left side. Surgery was
performed 0.5mo after the first visit. Arrowhead: Operculum.
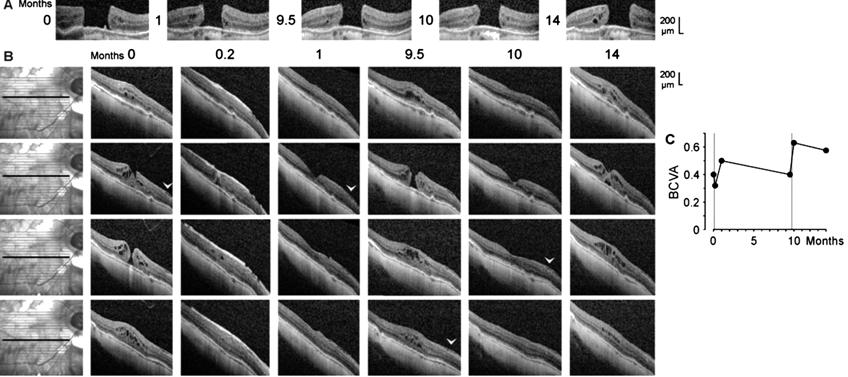
Figure 3 Closure of a macular hole after
vitrectomy with autologous platelet concentrate and reformation of the hole in
the right eye of patient
Patient 4 was a 63 year-old man who
presented, in the right eye, a fovea with a detached foveola which developed to
a lamellar macular hole and then to a full-thickness hole (Figure 4B). Vitrectomy
with ILM peeling and instillation of platelet concentrate was performed 8.6mo
after the first visit. Patient 5 was a 68 year-old woman with a
traction-induced full-thickness hole in the right eye (Figure 5). The surgery
included vitrectomy, removal of the epiretinal membrane, and instillation of
platelet concentrate. Patient 6 was a 56 year-old man with a full-thickness
hole in the left eye (Figure
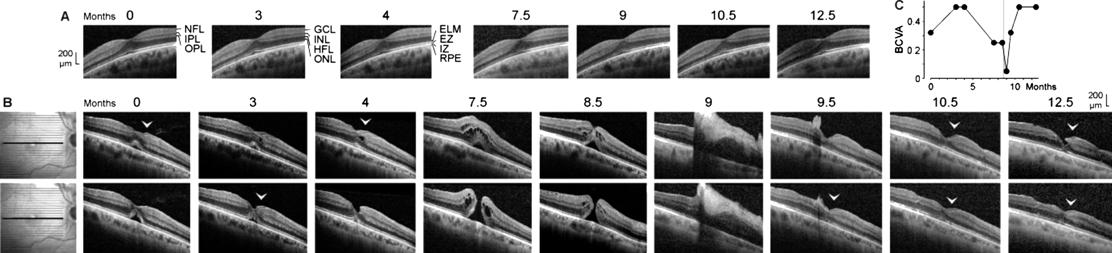
Figure 4 Development of a full-thickness
macular hole and restoration of the foveal structure followed by a formation of
a degenerative lamellar hole after vitrectomy with autologous platelet
concentrate in the right eye of patient 4 The months after the first examination
(0) are indicated above the images. A: Linear horizontal scans through the
fovea of the left eye. The fovea showed no structural abnormalities. B: Linear
horizontal scans through the fovea and parafovea of the right eye. The
orientations of the scans are shown at the left side. Surgery was performed
8.6mo after the first visit. The arrowheads indicate lamellar hole-associated
epiretinal proliferation. C: Time dependence of the BCVA. The vertical line
indicates the time of vitrectomy with instillation of autologous platelets.
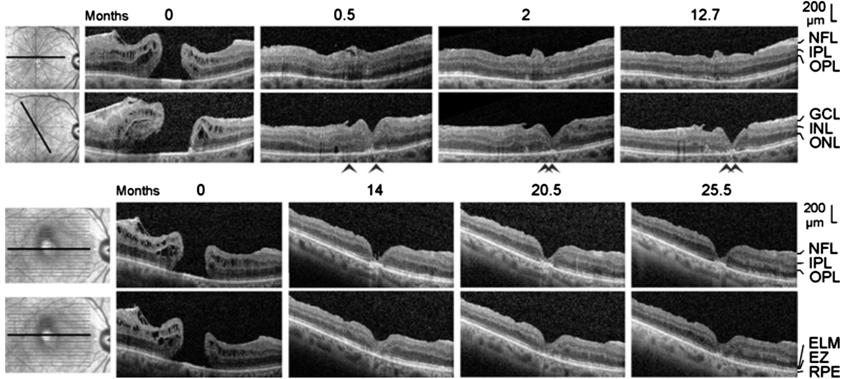
Figure 5 Closure of a macular hole after
vitrectomy with peeling of an epiretinal membrane and instillation of an
autologous platelet concentrate in the right eye of patient 5 The images show linear scans through the
fovea and parafovea; the orientations of the scans are shown at the left side.
The months after the first examination (0) are indicated above the images.
Surgery was performed one day after the first visit. The arrowheads indicate
sites of a direct contact between the central ONL and the RPE.
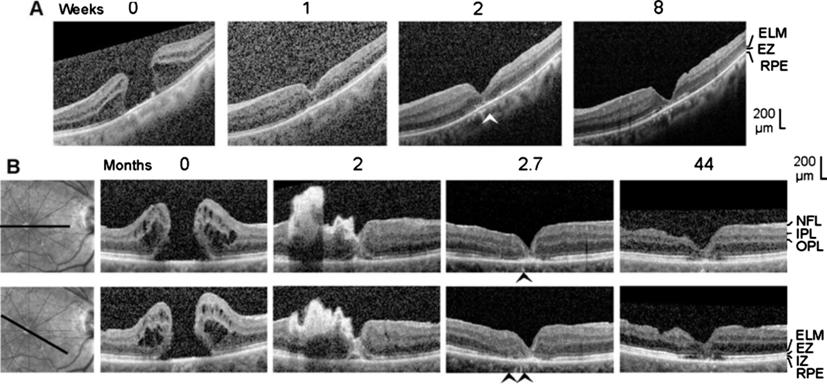
Figure 6 Closure of macular holes after
vitrectomy with autologous platelet concentrate in the left eye of patient 6
(A) and the right eye of patient 7 (B)
The images show linear scans through the fovea and parafovea; the
orientations of the scans are shown at the left side (B). The weeks (A) and
months (B), respectively, after the first clinical examination (0) are
indicated above the images. A: Surgery was performed one day after the first
visit. B: Revitrectomy with autologous platelet concentrate combined with
cataract surgery was performed 1.8mo after the first visit. The arrowheads
indicate sites of a direct contact between the central ONL and the RPE.
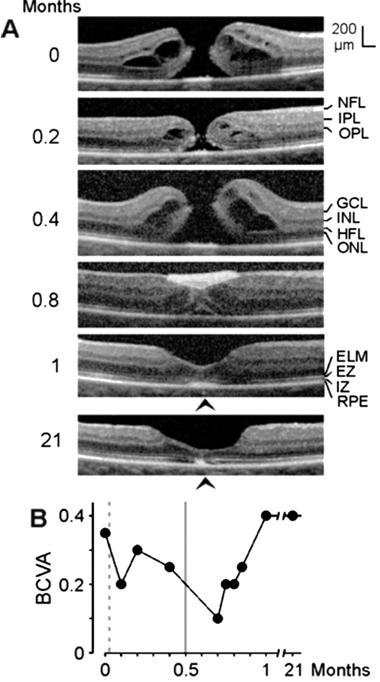
Figure 7 Closure of a macular hole after
revitrectomy with autologous platelet concentrate in the right eye of patient
We also reevaluated data of 34 eyes
of 33 patients (m/w, 2/31; mean±SD age, 70.9±7.4y) which were included in the
study of Degenhardt et al[27].
RESULTS
Patient 1 The full-thickness macular hole in
the right eye of patient 1 was formed by a disruption of the junction between
the inner layer of the foveola and the temporal foveal wall (arrowhead in
Figure
The hole did not close after
standard vitrectomy with ILM peeling which was performed 1.1mo after the first
visit (Figure
The RPE line, which is a light
reflection at the mitochondria-containing basal part of the RPE[28], showed no disruption during the development and
subsequent closure of the full-thickness hole (Figure
Figure
Patient 2 The full-thickness macular hole
(minimum diameter, 520 µm) in the left eye of patient 2 was formed by the
removal of a part of the inner Müller cell layer of the foveola (Figure 2B).
Cystic cavities between the OPL and HFL, and within the INL, ONL, and ganglion
cell layer (GCL), produced an elevation of the foveal walls associated with a detachment
of the central ONL from the RPE and EZ and IZ defects (Figure 2B). SD-OCT
images recorded 0.5mo after vitrectomy with ILM peeling and administration of
autologous platelet concentrate (1mo after the first examination) showed that
the hole was closed by platelets which were visible as a hyperreflective clot
that filled the center of the fovea (Figure 2B). The closure of the hole was
accompanied by a full resolution of the cystic cavities in the foveal tissue
(Figure 2B). This resulted in a drop of the elevated foveal walls and narrowing
of the walls around the platelet-filled foveal center which remained detached
from the RPE (Figure 2B). A further aggregate of hyperreflective platelets,
which caused shadows in the underlying retinal tissue, was attached at the
inner surface of the temporal parafovea (Figure 2B). This aggregate intruded
into the underlying retina and altered the layered structure of the tissue
(Figure 2B). One month after surgery (1.5mo after the first examinaton), the
hyperreflective platelets disappeared (Figure 2B). At this time, the central
foveal walls fused and the horizontal gaps in the central ONL and ELM were
closed; however, the central ONL was still detached from the RPE and there was
no foveal pit (Figure 2B). The preoperative BCVA was 0.25; the BCVA decreased
to 0.16 at one month, and increased to 0.25 at 1.5mo.
Patient 3 SD-OCT scans of the fovea of the
untreated left eye of patient 3 showed a stage-4 macular hole (minimum
diameter, 610 µm; Figure
Shortly after vitrectomy with
instillation of autologous platelets performed one day after the first visit,
the platelets were visible as a hyperreflective tissue which filled the hole
and which lay above the inner surface of the parafovea (Figure 3B). The
platelet-assisted closure of the hole was accompanied by a resolution of most
cystic cavities in the foveal tissue (Figure 3B). One month after the first
examination, the platelets fully disappeared, and the central fovea reattached
at the RPE; this was associated with the reformation of the foveal pit (Figure
3B). Within 9.5mo, the macular hole developed again (minimum diameter, 220 µm),
likely by traction exerted by the epiretinal membrane (Figure 3B). After a
second administration of platelet concentrate 9.7mo after the first
examination, the hole closed again; this was associated with the regeneration
of the central defect of the ELM line, but not of the EZ line (Figure 3B). Four
months later, the reformation of cystic cavities in the foveal tissue resulted
in a renewed elevation of the foveal walls and detachment of the central ONL
(Figure 3B). As shown in Figure
Patient 4 SD-OCT images of the fovea of the
right eye of patient 4 recorded at the first examination showed a detachment of
the foveola from the RPE; this was associated with a shallow foveal pit and a
loss of the integrity of the central photoreceptors, as indicated by the
central defect of the EZ line (Figure 4B). In addition, there was a lamellar
macular hole-associated epiretinal proliferation (LHEP) at the vitreal surface
of the nasal foveal wall (Figure 4B) which may suggest that a degenerative
lamellar hole[29] was present before the first
examination.
During the following 3mo, the EZ
line partly recovered (Figure 4B). This was associated with an increase of BCVA
(Figure
Vitrectomy with administration of
platelet concentrate was performed 8.6mo after the first visit. Shortly after
surgery, a large aggregate of hyperreflective platelets adhered at the inner
surface of the fovea and the nasal parafovea which caused shadows in the
underlying retinal tissue (Figure 4B) and a strong decrease of the BCVA (Figure
Patient 5 SD-OCT scans of the fovea of the
right eye of patient 5 recorded at the first visit showed a large
full-thickness hole (minimum diameter, 650 µm; Figure 5). There was an
epiretinal membrane in the dorsotemporal parafovea (Figure 5) which likely
caused the development of the hole. The hole closed within 0.5mo after
vitrectomy with removal of the epiretinal membrane and instillation of platelet
concentrate; the closure was associated with a resolution of the cystic
cavities in the foveal walls (Figure 5). There were sites of a direct contact
between the central ONL and RPE (as indicated by the absence of the ELM and EZ
lines; arrowheads in Figure 5). In the further course, a regular foveola was
not formed. Between 0.5 and 14mo after the first examination, there was a
time-dependent increase in the horizontal gap of the central ONL associated
with a deepening of the foveal pit and a thinning of the foveal center (Figure
5). There remained a gap in the central ONL until the end of the examination
period (Figure 5). In addition, the defects of the central ELM, EZ, and IZ
lines remained, and there was a thickening of the RPE line in the center of the
fovea (Figure 5). These signs indicate that there were no photoreceptors in the
foveal center after closure of the hole. The absence of an ONL in the foveal
center was associated with the presence of a very deep foveal pit; the base of
the pit was at the level of the ELM (Figure 5). The foveal center was filled by
a tissue of medium reflectivity, likely Müller cells, and hyperreflective RPE
cells (Figure 5). The preoperative BCVA was 0.1. Within 2mo after surgery, the
BCVA increased to 0.16 and remained at this value until the end of the
examination period.
Patient 6 The first clinical examination
revealed the presence of a large macular hole (minimum diameter, 690 µm) in the
left eye of patient 6 (Figure
Patient 7 Six months before the first visit,
an unsuccessful standard vitrectomy with ILM peeling was performed to close a
full-thickness macular hole in the the right eye of patient 7. SD-OCT scans of
the fovea recorded at the first visit showed a large full-thickness hole
(minimum diameter, 590 µm) which was surrounded by foveal walls that were highly
elevated and contained large cystic spaces (Figure 6B). After vitrectomy with
platelet concentrate performed 1.8mo after the first visit, the hole had a
smaller diameter because the foveal walls dropped down as a result of the
resolution of the cystic cavities. Hyperreflective platelets filled the hole
and were attached at the inner surface of the temporal foveal wall and
parafovea (Figure 6B). The platelets disappeared between 2 and 2.7mo (Figure
6B). The foveal tissue was reattached at the RPE; however, there were areas
with a direct contact between the central ONL and RPE as indicated by the
absence of ELM and EZ lines (arrowheads in Figure 6B). In addition, the RPE
line displayed a central thickening (Figure 6B). After 44mo, the foveal
structure was similar as at 2.7mo: there was no regular foveola, the foveal
center was filled by a tissue composed of medium-reflective Müller cells and
hyperreflective RPE cells, the central RPE line was thickened (although the
width of the thickened central RPE line decreased between 2.7 and 44mo), and
there were no photoreceptors in the foveal center as indicated by the defects
of the ELM, EZ, and IZ lines (Figure 6B). In addition, the inner layers of the
parafovea displayed degenerative alterations (Figure 6B). The BCVA increased
from 0.08 before vitrectomy with platelet concentrate to 0.2 at the end of the
examination period.
Patient 8 SD-OCT scans recorded at the first
clinical examination showed the presence of a macular hole (minimum diameter,
120 µm) in the right eye of patient 8 (Figure
Data of Further Patients We recently described the anatomical
and functional outcome after revitrectomy with application of autologous
platelet concentrate in eyes with idiopathic macular hole[27].
A reevaluation of the data of 34 eyes of 33 patients, which were included in
this study and which presented a successful surgery with a closure of the hole,
showed in 16 eyes a regular regeneration of the central fovea and in 18 eyes an
irregular regeneration. Figure
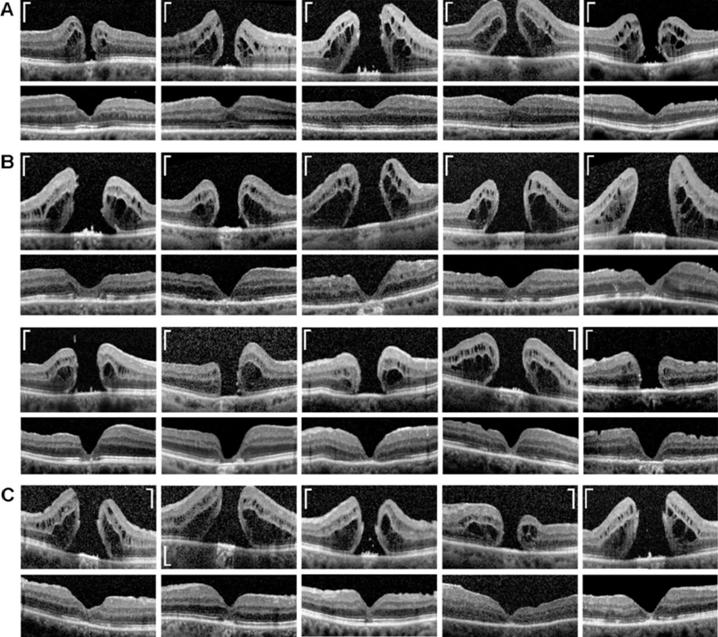
Figure 8 Examples of regular (A),
irregular (B), and mixed type regeneration (C) of the central fovea after
closure of full-thickness holes by (re)vitrectomy and autologous platelet
concentrate The images show
linear scans through the fovea and parafovea of different patients.
Preoperative scans are shown above; postoperative scans obtained between 1 and
55mo after surgery are shown below. Scale bars, 200 µm.
DISCUSSION
Autologous platelet concentrate is a
safe and effective adjunct to the surgical management of macular holes[15-18]. We investigated with SD-OCT
the regeneration of the foveal structure in 8 eyes of 8 patients which
underwent (re)vitrectomy with autologous platelet concentrate to close a
full-thickness macular hole. We found different modes of foveal regeneration
after hole closure. A regular regeneration resulted in the formation of a fovea
which contained a foveola and photoreceptors in the center (Figure
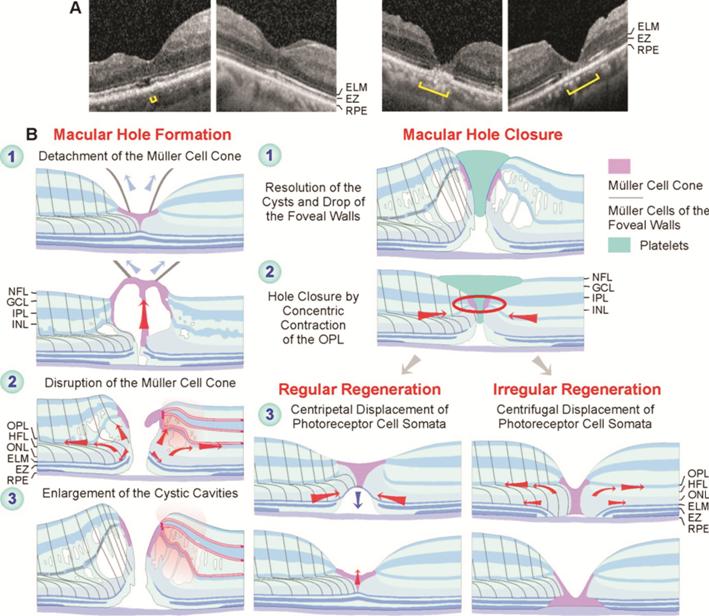
Figure 9 Regular and irregular
regeneration of the fovea after vitrectomy with instillation of autologous
platelet concentrate to stimulate the closure of a full-thickness macular hole A: Final foveal structure after hole
closure in the eyes of patients 1, 4, 5, and 6 (from left to right). The foveas
of patients 1 and 4 regenerated regularly while the foveas of patients 5 and 6
regenerated irregularly. Brackets: EZ defect. B: Putative mechanisms of macular
hole formation and regular and irregular foveal regeneration after
platelet-assisted closure of macular holes.
The mechanisms which mediate the
closure of macular holes are incompletely understood. Because the fovea is free
of astrocytes[3], the closure is likely mediated
by Müller cells. Normally, Müller cells maintain the structural stability of
the fovea[2-3]. Müller
cell-mediated tissue movements may be involved in the regeneration of the
foveal shape after surgical or spontaneous closure of macular holes[8-9]. The cardinal event of hole closure
is the sealing of the horizontal gap in the central ONL[9].
The spontaneous closure of small holes was suggested to be mediated by two
tissue movements exerted by Müller cells of the foveal walls: 1) a concentric
contraction of the Müller cell side processes in the OPL and 2) a concentric
contraction of the Müller cell processes which envelop the photoreceptor cells
at the ELM resulting in a centripetal shift of the central photoreceptor cell
somata[9]. Both movements cause a centripetal
shift of the foveal walls, a narrowing and fusion of the remnants of the Müller
cell cone, and the closure of the hole at the level of the OPL/inner part of
the ONL[9]. Similar Müller cell-mediated tissue
movements seem to contribute to the hole closure and the regeneration of a
regular central fovea after platelet-assisted surgery (Figure 9B).
The spontaneous closure of small
holes is followed by the regeneration of a regular central fovea, i.e.,
the recreation of a foveola, the centripetal shift of photoreceptor cell
somata, and the regeneration of the central photoreceptor segments[9]. However, there may be a considerable time delay
between the closure of the hole and the begin of the restoration of the regular
foveal shape[9]. A time delay of about 5mo was
also observed after platelet-assisted closure of the hole in patient 1 (Figure
We suggested that the spontaneous
closure of small macular holes proceeds by Müller cell-mediated tissue
movements, without cell proliferation[9]. This was
likely also the case of the platelet-assisted closure of macular holes and the
regular foveal regeneration. On the other hand, the irregular regeneration of
the foveal structure was mediated in part by proliferation of Müller and RPE
cells. In these cases, the hole closed by (at least) two mechanisms: the
resolution of the cystic cavities, which resulted in a drop of the elevated
foveal walls around the hole, and Müller cell-mediated centripetal tissue
movements. Thereafter, when the horizontal gap in the central ONL was not
closed or increased again by a centrifugal displacement of the photoreceptor
cells, the foveal center was filled by a tissue formed by proliferating Müller
and RPE cells (Figure 9B).
It was suggested that the stimulatory
effect of platelets on the surgical closure of macular holes depends on the
release of growth factors[19] which stimulate the
proliferation and migration of Müller and RPE cells[22-26]. It could be that platelet-derived growth factors
stimulate the proliferation of Müller and RPE cells in cases of irregular
foveal regeneration. However, in two cases, the platelet concentrate was
already removed for a relatively long time period until the onset of Müller and
RPE cell proliferation (2wk in patient 6 and at least 12.7mo in the case of
patient 5); thus, it seems to be rather unlikely that the cell proliferation in
these cases was only stimulated by platelet-derived growth factors. The
findings may suggest that platelets stimulate the closure of a hole, which is
likely mediated by the resolution of the cystic cavities, which causes a drop
of the elevated foveal walls, and a concentric contraction exerted by the
Müller cells of the foveal walls[9], whereas the
regeneration of the foveal morphology, which may proceed with a considerable
time delay after hole closure, is likely not stimulated by platelets.
It is unclear which cellular and
molecular events trigger the regular and irregular regeneration of the fovea.
One possibility might be the presence or absence of remnants of the disrupted
Müller cell cone; the latter may prevent a regular regeneration of the fovea[9]. Another possibility is the presence or absence of a
direct contact between the central ONL and RPE after the closure of the hole.
The direct contact between the central ONL and RPE (Figures 5,
The regular foveal regeneration
after hole closure involved an increase in the thickness of the central ONL
mediated by a centripetal movement of photoreceptor cells (Figure 9B). In cases
of irregular foveal regeneration, the gap in the central ONL was not closed or
increased again after hole closure, by a centrifugal movement of photoreceptor
cells. It was suggested that the centripetal movement of photoreceptor cells
during the regular regeneration of the fovea is mediated by a concentric
contraction of the Müller cell processes which envelop the photoreceptor cells
in the outer ONL and at the ELM (Figure 9B)[9]. It
could be that the direct contact between the central ONL (i.e., the
outer processes of Müller cells of the foveal walls which envelop the
photoreceptor cell somata in the ONL) and the RPE cells inhibits the
centripetal movement of the Müller cell processes; instead, the Müller cell
processes may retract from the foveal center. The retraction of the Müller cell
processes from the foveal center causes the centrifugal shift of the
photoreceptor cell somata which results in a widening of the gap in the central
ONL.
A prominent feature of the
platelet-assisted closure of macular holes observed in all investigated
patients is the rapid resolution of the cystic cavities in the foveal walls. A
similar rapid resolution of cystic cavities was observed after spontaneous
closure of small macular holes[9]. These findings
support the hypothesis of a cystic genesis of macular holes[30].
The cysts produce the elevation of the foveal walls around the hole which is
associated with a widening of the hole, a detachment of the central ONL, and
the formation of a gap in the ONL of the foveola (Figure 9B). Redevelopment of
the cystic cavities after hole closure may result in a reopening of the hole
(Figure 3B). The cystic cavities are likely result from a leakage of the foveal
avascular zone-delimitating vessels in the foveal walls[31].
A dysfunction of Müller cells may contribute to the development of edematous
cysts, due to a dysregulated fluid clearance[32].
Mechanical stress resulting from vitreofoveal traction or contraction of
epiretinal membranes may induce a dysfunction of Müller cells; mechanical stress
exterted on the tissue by the hydrostatic pressure within the increasing cysts
may further induce Müller cell dysfunction.
The present data suggest that the
reformation of a regular foveola after hole closure proceeds by a Müller
cell-mediated displacement of photoreceptor cells towards the foveal center. A
similar Müller cell-mediated movement of photoreceptor cell somata contributes
to the ontogenetic development of the foveola[3].
The developmental displacement of the photoreceptor cell somata proceeds after
birth and is associated with an elongation and thinning of the central
receptors, and a thickening of the ONL in the foveola[33].
The latter proceeds up to 17-25y after birth[34],
and the increase of the central receptor density improves the vision until 21
years of age[35]. Because the centripetal
displacement of the photoreceptor cell somata continues for many years after
birth, it seems to be conceivable that a similar mechanism contributes also to
the reformation of a regular foveola after macular hole closure[9].
Platelets may support the closure of
macular holes by various mechanisms, including 1) the factors released from
platelets[19] may stimulate the resolution of the
cystic cavities in the foveal walls and the concentric contraction of Müller
cell processes in the OPL which both contribute to hole closure (Figure 9B); 2)
platelet aggregates may provide a scaffold and may stimulate the production of
extracellular matrix with junction sites for contracting Müller cell processes;
and 3) platelets which fill the hole (Figures 2B, 3B, and 6B) may decrease the
diameter of the hole to values smaller than 400 µm at which the hole may close
spontaneously[10-11].
Platelet-derived growth factors are known to stimulate the contraction of
Müller cells[36-37] and thus
may induce Müller cell-mediated tissue movements implicated in hole closure[9].
The main limitations of the present
study are the small sample size and the restrospective design at one
institution. Further investigation of the course of the platelet-assisted hole
closure and the subsequent foveal regeneration will be required for a better
understanding of the factors and cellular mechanisms which drive regular and
irregular regeneration of the foveola, including the question whether the
contact between RPE cells and Müller cells of the foveal walls plays a role in
inhibition of regular regeneration.
In summary, we describe different
modes of foveal regeneration after closure of full-thickness macular holes by
(re)vitrectomy with usage of autologous platelet concentrate. The foveal
regeneration can proceed with or without a temporary detachment of the foveal
center from the RPE, and with or without a contact between the central ONL and
the RPE. Contacts between the central ONL and RPE may prevent a regular foveal
regeneration, and the center of the fovea is only formed by Müller and RPE
cells and does not contain photoreceptors. A regular regeneration, which
results in the formation of a fovea with a foveola and photoreceptors in the
center, is likely produced by Müller cell-mediated tissue movements whereas an
irregular regeneration proceeds in part by proliferation of Müller and RPE
cells. An irregular regeneration of the foveal structure, with the absence of
photoreceptors in the foveal center, may be one reason for a rather moderate
postoperative improvement of the visual acuity despite successful closure of
the hole[27]. On the other hand, the decrease in
the size of the photoreceptor-free area after hole closure due to the
resolution of cystic cavities, which allows a reattachment of the foveal walls
at the RPE, may decrease the size of the central scotoma.
ACKNOWLEDGEMENTS
Authors’ contributions: Bringmann A and Wiedemann P designed
the experiments. Jochmann C, Unterlauft JD, Wiedemann R, and Rehak M performed
the experiments. Bringmann A performed the data analysis and wrote the paper.
Wiedemann P revised the paper.
Conflicts of Interest: Bringmann A, None; Jochmann
C, None; Unterlauft JD, None; Wiedemann R, None; Rehak M,
None; Wiedemann P, None.
REFERENCES