
·Clinical Research·
Comparison
of two different treatment regimens’ efficacy in neovascular age-related
macular degeneration in Turkish population—based on real life data-Bosphorus
RWE Study Group
Burak
Erden1, Selim Bölükbaşı1,
Abdullah Özkaya2,
Levent Karabaş3,
Cengiz Alagöz4,
Zeynep Alkın4,
Özgür
Artunay4, Sadık Etka Bayramoğlu5,
Gökhan
Demir4, Mehmet Demir6, Ali Demircan4, Gürkan
Erdoğan4,
Mehmet Erdoğan6,
Erdem Eriş4,
Havva Kaldırım7,
İsmail
Umut Onur8, Özen Ayrancı
Osmanbaşoğlu9,
Sezin Özdoğan
Erkul9, Mine Öztürk10,
İrfan
Perente4, Kübra Sarıcı5,
Nihat Sayın5,
Dilek Yaşa4,
İhsan Yılmaz4,
Zeynep Yılmazabdurrahmanoğlu7;
Bosphorus Retina Study Group
1Ophthalmology
Clinic, Okmeydanı Training and Research Hospital, Istanbul 34384, Turkey
2Ophthalmology
Clinic, Memorial Şişli Hospital, Istanbul 34384, Turkey
3Department
of Ophthalmology, Kocaeli University Faculty of Medicine, Kocaeli 41380, Turkey
4Ophthalmology
Clinic, Beyoğlu Eye Training and Research Hospital, Istanbul 34421, Turkey
5Ophthalmology
Clinic, Kanuni Sultan Süleyman Training and Research Hospital, Istanbul 34303,
Turkey
6Ophthalmology
Clinic, Şişli Etfal Training and Research Hospital, Istanbul 34360, Turkey
7Ophthalmology
Clinic, Bağcılar Training and Research Hospital, Istanbul 34200, Turkey
8Ophthalmology
Clinic, Bakırköy Dr. Sadi Konuk Training and Research Hospital, Istanbul 34147,
Turkey
9Ophthalmology
Clinic, Istanbul Training and Research Hospital, Istanbul 34098, Turkey
10Ophthalmology
Clinic, Haseki Training and Research Hospital, Istanbul 34096, Turkey
Correspondence to: Burak Erden. Şelale Cad. Manolya Evleri B1 Blok D 27,
Bahçeşehir/Istanbul 34488, Turkey. drburakerden@gmail.com
Received:
Abstract
AIM: To compare two
different anti-vascular endothelial growth factor (anti-VEGF) treatment
regimens’-a priori pro re nata (PRN) and PRN regimen following the
loading phase-anatomical and functional results in neovascular age-related
macular degeneration (nAMD) patients.
METHODS: Totally 544 nAMD
patients followed and treated with aflibercept (n=135) and ranibizumab (n=409)
at 9 different centers between 2013 and 2015 were enrolled into this
retrospective multicenter study. Patients with initial best corrected visual
acuity (BCVA) interval of 1.3-0.3 (logMAR) and a minimum follow-up of 12mo were
included. Patients under two different regimens-a priori pro re nata
(1+PRN) or 3 consecutive intravitreal injections followed by a PRN regimen
(3+PRN)-were compared in BCVA at 3th, 6th and 12th
months, and in central macular thickness (CMT) at 6th and 12th
months. The total study group, intravitreal ranibizumab (IVR) and intravitreal
aflibercept (IVA) groups were evaluated separately.
RESULTS: The mean CMT
decreased in the 1+PRN (n=101) regimen from 407 to 358 and 340 µm and in
the 3+PRN (n=443) group from 398 to 318 and finally to 310 µm at months
6 and 12, respectively. Anatomically, the CMT reduction at 6th month
(48.5 vs 76.4; P<0.05) was statistically significant in favor
of 3+PRN group. BCVA changed in 1+PRN group from 0.77 to 0.78, 0.75 and 0.75;
in 3+PRN group from 0.81 to 0.69, 0.72, and 0.76 at months 3, 6, and 12,
respectively. Visual gain was statistically better in 3+PRN group at 3th
month (-0.01 vs 0.12; P<0.001). In IVR group, CMT reduction
was in greater in 3+PRN at 6th (44 vs 72) and 12th
month (61 vs 84), but statistically insignificant. The 3+PRN group
revealed statistically better visual results at 3th month (-0.02
vs 0.11, P<0.05). In IVA group, although statistically insignificant,
CMT reduction (61 vs 89, 6th month; 85 vs 97, 12th
month) and visual gain (0.02 vs 0.16; 0.02 vs 0.14; 0.05 vs
0.11) was found in favor of 3+PRN group at all visits.
CONCLUSION: The loading dose of
anti-VEGF treatments in nAMD leads to significantly better anatomical and
functional results, regardless of the agent, specially in early follow-up
interval.
KEYWORDS: aflibercept;
neovascular age-related macular degeneration; ranibizumab; loading dose;
treatment regimen
DOI:10.18240/ijo.2020.01.15
Citation: Erden B, Bölükbaşı S, Özkaya A,
Karabaş L, Alagöz C, Alkın Z, Artunay Ö, Bayramoğlu SE, Demir G, Demir M,
Demircan A, Erdoğan G, Erdoğan M, Eriş E, Kaldırım H, Onur İU, Osmanbaşoğlu ÖA,
Özdoğan Erkul S, Öztürk M, Perente İ, Sarıcı K, Sayın N, Yaşa D, Yılmaz İ,
Yılmazabdurrahmanoğlu Z; Bosphorus Retina Study Group. Comparison
of two different treatment regimens’
efficacy in neovascular age-related macular degeneration in Turkish population—based
on real life data-Bosphorus RWE Study Group. Int J Ophthalmol 2020;13(1):104-111
INTRODUCTION
Age-related macular degeneration (AMD) is the most common
cause of vision loss and legal blindness in the population with age over 60y in
the developed countries[1-2].
The AMD is divided into two major clinical subgroups according to the
presence or absence of a choroidal neovascular membrane (CNVM); dry AMD
subgroup starts with the developing of drusen in the posterior pole, progresses
in severe cases to geographical atrophy at the fovea leading to severe visual
impairment, the neovascular subgroup on the other hand is landmarked with the
presence of a CNVM of various presentations. Although only 10%-20% of AMD
patients are of neovascular type, it is responsible for severe vision loss or
blindness in approximately 90% of AMD cases. Historically, photodynamic therapy
was the first critical milestone to stop the progression of neovascular AMD
(nAMD) at the beginning of this century[3].
Intravitreal pegaptanip sodium was first anti-vascular endothelial growth
factor (anti-VEGF) drug introduced into the AMD treatment armamentorium[4]. Its promising results compared to the standard care
were followed by off-label bevacizumab[5],
on-label ranibizumab and aflibercept in search for better clinical outcomes for
this devastating disease. Nowadays, anti-VEGF therapy is the most effective
treatment option of nAMD.
Several pivotal clinical trials[6-8] suggested strict monthly treatment regimens for
intravitreal anti-VEGF administration, accompanied with a close monitoring in
nAMD patients[4,6], but in
clinicians’ daily praxis this strict protocol mostly failed due to several
technical reasons. The economical and social burden of such a chronic therapy,
the over-loading effect of endless treatment sessions and monitoring visits on
patients and retinal physicians inhibited all participants from practicing
monthly regimens. In the current study, we aimed to evaluate the treatment
outcomes from nine tertiary retinal centers and to investigate the effect of
initial regimen preferences based on our real life experience.
SUBJECTS AND METHODS
Ethical Approval
Written informed consent was
obtained from all patients before all invasive procedures in the follow-up and
the study adhered to the tenets of the Declaration of Helsinki. Ethical board
approval was obtained from Faculty of Medicine, Kocaeli University.
This is a multicenter, retrospective, observational,
comparative real-life experience study, conducted in 9 tertiary centers in
Istanbul and Kocaeli/Turkey. The records of treatment naive nAMD patients who
were treated for the first time with an anti-VEGF—either ranibizumab or
aflibercept-agent between January 2013 and December 2015 were reviewed by the
investigators. Patients were divided into two major groups according to their
treatment initiation regimen. The patients who were started with a priori pro
re nata (PRN; as needed) treatment regimen were contributed into the 1+PRN
group, on the other hand, patients who underwent a loading phase with three
consecutive injections followed by a PRN regimen were enrolled into the 3+PRN
group.
Patient Enrollment and Follow-up Our major inclusion criteria were being age of ≥50y, a
diagnoses of nAMD, a minimum follow-up time of 12mo and having a baseline best
corrected visual acuity (BCVA) within the range of 1.3
All eligible patients underwent comprehensive
ophthalmological examination including BCVA measurement in Snellen ratios or
the Early Treatment Diabetic Retinopathy Study (ETDRS) letters, slit-lamp
biomicroscopy and fundus examination, and intraocular pressure measurement via
Goldmann applanation tonometry at pretreatment, months 3, 6 and 12 visits.
Fluorescein angiography (FA), and spectral domain optical coherence tomography
(SD-OCT) imaging were performed before treatment initiation and OCT examination
was repeated at all centers at months 3, 6 and 12. Due to the multicenter
nature of this study, several brands of FA and SD-OCT devices were utilized to
evaluate the study population. All prescheduled examinations were planned in
the study groups on a monthly or bimonthly basis, except for FA. FA was
repeated in the follow-up depending on the physician’s individual clinical
decision—only when a new and unexpected clinical symptom has arisen. SD-OCT was
used mainly for measurement of central macular thickness (CMT) values. CMT was
defined as the mean thickness of the neurosensory retina in
Drug Administration
All intravitreal injections were
administered under sterile conditions. Following topical anesthesia and surface
disinfection with 5% povidone-iodine, intravitreal 0.5 mL/0.1 mL ranibizumab or
2 mg/0.1 mL aflibercept were injected through the pars plana 3.5
Statistical Analysis
Following the data collection
from all these tertiary centers , all BCVA values were converted into logMAR
for statistical purposes. The data were evaluated for normality using the
Kolmogorov-Smirnov test. As the distribution of the BCVA and CMT values were
found to be normal, changes in these parameters between baseline and following time
points were assessed with repeated measures test. Student’s t-tests and
repeated measures of ANOVA were preferred for inter-group and intra-group
statistical analyses using SPSS (Version 22.0, SPSS Inc., Chicago, IL, USA). An
overall 5% type-1 error level was considered to be statistically significant.
RESULTS
Five hundred and forty-four patients (135 IVA; 409 IVR)
diagnosed with nAMD were enrolled into the study according to our
inclusion/exclusion criteria. The mean age was 73.74±8.6y (range 50-94y); 309
patients (56.8%) were men and 235 (43.2%) were women. Ninety-four eyes (15.7%)
had been treated with either bevacizumab or ranibizumab before, while 450 eyes
(82.7%) were defined as treatment naïve. In the total study population; 409
(75.2%) eyes were treated with 0.5 mg IVR, whereas 135 (24.8%) eyes were
received 2 mg IVA therapy. According to the treatment regimen, 101 eyes (18.6%)
were included into the 1+PRN arm, 443 eyes were enrolled into the 3+PRN arm of
the study. Both arms were statistically comparable in the means of age, gender
distribution, baseline visual acuity and CMT values. Baseline characteristics
of the study population is summarized at Table 1.
Table 1 Baseline characteristics of both study arms were
similar in means of age, gender distribution and mean values of visual acuity
and CMT
|
Parameters |
1+PRN
group (n=101) |
3+PRN
group (n=443) |
P |
|
Age (y) |
74.7±9.2 |
73.5±8.5 |
0.24 |
|
Gender (n; M/F) |
79/56 |
230/179 |
0.71 |
|
Baseline CMT (µm) |
407±134 |
398±138 |
0.54 |
|
Baseline BCVA (logMAR) |
0.77±0.34 |
0.81±0.32 |
0.19 |
CMT: Central macular thickness; BCVA: Best corrected
visual acuity.
All patients had a minimum follow-up interval of 12mo. No
systemic complication was reported in this one-year follow-up. Ocular complications
were limited to punctate epitheliopathy (n=17, 3.1%), subconjunctival
hemorrhage (n=48, 8.8%) and mild anterior chamber reaction (n=22,
4%). Severe complications such as endophthalmitis or retinal detachment were
not encountered in any of the eyes during the study period.
Functional Results
The mean baseline BCVA changed in
1+PRN group (n=101) of the total study population from 0.77±0.34 to
0.78±0.45 (month 3; P=0.79), 0.75±0.45 (month 6; P=0.58) and
0.75±0.44 (month 12; P=0.65). In the 3+PRN group (n=443),
however, the mean BCVA increased significantly from 0.81±0.32 to 0.69±0.32
(month 3; P<0.001), 0.72±0.43 (month 6; P<0.001) and
0.76±0.46 (month 12; P=0.006). When the effect of treatment regimen on
the visual results at all time points was analyzed, the 3+PRN group was found
significantly superior over 1+PRN group in the follow-up (repeated measures; P=0.005;
Figure 1). The most significant visual gain difference was found in favor of
3+PRN group at 3rd month visit (-0.01 vs 0.12; P<0.001).
The mean numbers of injections (2.4 vs 4.4; P<0.01) and visits
(6.4 vs 7.2; P<0.01) were significantly higher in the 3+PRN
arm of the study population.
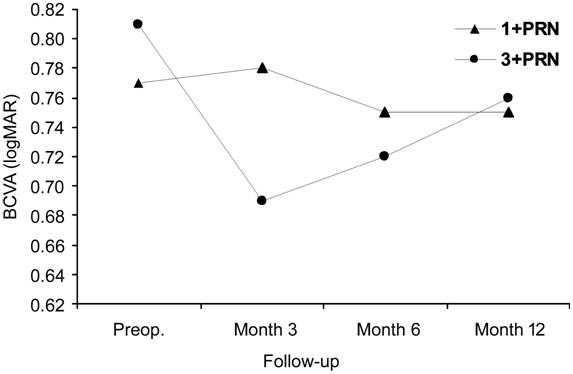
Figure 1 The comparison of visual gain between treatment
groups in the total study population revealed a significant superiority of
3+PRN regimen over the 1+PRN approach (P=0.005).
The total study population was divided then into two
subgroups according to the type of anti-VEGF agent. Treatment regimens’ visual
outcomes were analyzed in IVR and IVA subgroups separately. In IVR subgroup (n=409),
the mean BCVA of the 1+PRN arm (n=75) changed insignificantly from
baseline 0.77±0.33 to 0.80±0.44 (month 3; P=0.60), 0.76±0.45 (month 6; P=0.68)
and 0.77±0.44 (month 12; P=0.90). In 3+PRN arm (n=334), however,
BCVA improved significantly from baseline value of 0.80±0.32 to 0.70±0.37
(month 3; P<0.001), 0.72±0.42 (month 6; P<0.001) and
0.76±0.46 at the final visit (month 12; P=0.09 ). The visual gain
comparison of different treatment arms in IVR subgroup revealed a statistically
significant difference in favor of 3+PRN arm (repeated measures; P=0.003;
Figure 2). The mean number of intravitreal treatments in one year was also
found significantly higher in the 3+PRN (4.67 vs 2.97; P<0.001),
although the mean numbers of visits demonstrated no significant difference in
1+PRN and 3+PRN arms (6.7 vs 7.1; P=0.19; respectively).
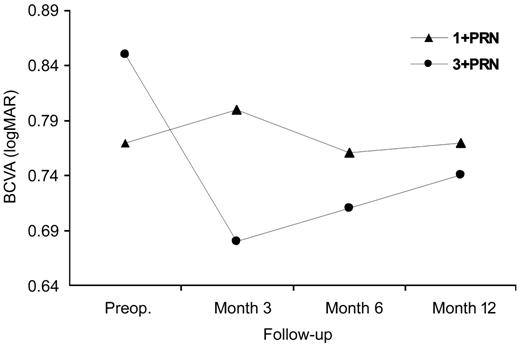
Figure
The visual outcome analyses in the IVA (n=135)
subgroup revealed similar results in favor of 3+PRN regimen such as in the IVR
subgroup. In 1+PRN arm (n=26), mean BCVA changed insignificantly from
0.75±0.36 (baseline) to 0.73±0.45 (month 3; P=0.70), 0.72±0.46 (month 6;
P=0.69) and finally to 0.70±0.45 (month 12; P=0.47). The BCVA in
3+PRN arm (n=109) increased significantly from 0.85±0.33 (baseline) to
0.68±0.37 (month 3; P<0.001), 0.71±0.45 (month 6; P<0.001)
and 0.74±0.49 (month 12; P=0.009). However, the comparison within the IVA
subgroup revealed no significant difference between 3+PRN and 1+PRN arms
(repeated measures; P=0.068; Figure 3). Although the mean number of
visits in the study period were similar in 1+PRN and 3+PRN IVA arms (7.1 vs
7.5, respectively; P=0.47), the mean number of intravitreal
administrations differed from each other significantly (2.4 vs 4.2,
respectively; P<0.001).
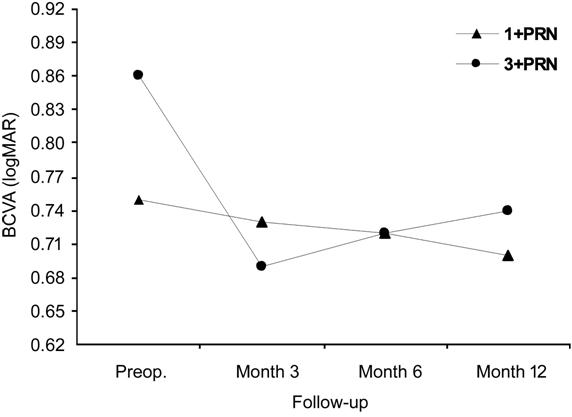
Figure 3 The visual results in IVA subgroup were in favor
of 3+PRN arm, but did not reach statistical significance (P=0.068).
Anatomical Outcomes
The mean CMT value in the 1+PRN
arm (n=101) of the total study population decreased significantly from
baseline value of 407±134 µm to 358±111 µm (month 6; P<0.001) and to
340±111 µm (month 12; P<0.001) at the final visit. Likewise, in the
3+PRN arm the mean CMT was reduced significantly from 398±138 µm to 318±103 µm
(month 6; P<0.001) and finally to 310±101 µm (month 12; P<0.001).
The statistical between-group comparison in aspect of anatomical gain showed
that there was no significant difference between these two regimens (repeated
measures; P=0.08; Figure 4).
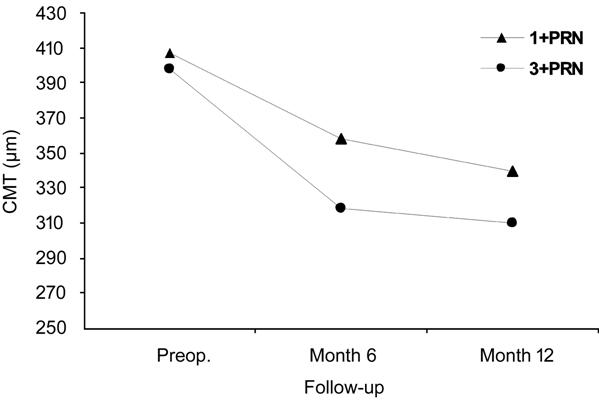
Figure 4 Both regimens in the total study population
resulted in comparable anatomical gains in means of CMT reduction (P=0.08).
In the sub-analyses of anti-VEGF agent based subgroups;
the mean CMT in IVR 1+PRN arm (n=75) declined significantly from 395±124
µm (baseline) to 351±112 µm (month 6; P<0.001) and to 334±113 µm
(month12; P<0.001). Meanwhile, the baseline CMT in IVR 3+PRN arm (n=334)
decreased also significantly from 398±135 µm (baseline) to 315±100 µm (month 6;
P<0.001) and to 307±103 µm (month 12; P<0.001). The CMT
gain analyses between 1+PRN and 3+PRN arms within IVR subgroup revealed no
significant difference (repeated measures; P=0.14; Figure 5).
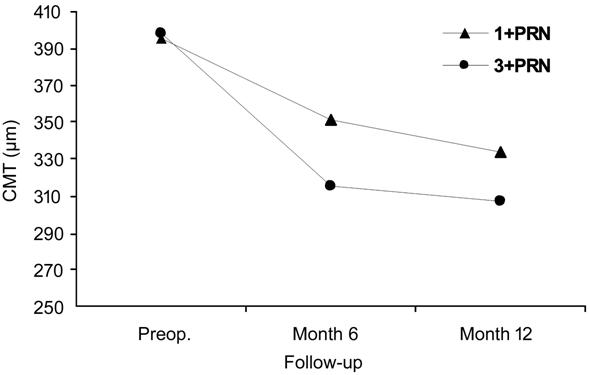
Figure 5 The CMT reduction was found statistically
comparable in both regimen arms of the IVR group (P=0.14).
In IVA subgroup; the mean CMT value of 1+PRN arm (n=26)
was reduced significantly from 444±155 µm (baseline) to 380±107 µm (month 6; P=0.019)
and finally to 356±136 µm (month 12; P=0.018). In 3+PRN arm (n=109)
the CMT declined very significantly from 421±144 µm (baseline) to 330±112 µm
(month 6; P<0.001) and to 321±98 µm (month 12; P<0.001).
The anatomical gain comparison between 1+PRN and 3+PRN arms within IVA subgroup
was analyzed and no statistical difference was found through the study period
regarding the treatment regimen (repeated measures; P=0.47; Figure 6).
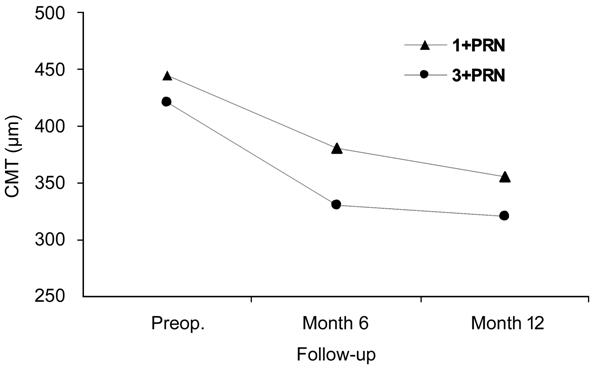
Figure 6 The CMT reduction in the IVA subgroup was
similar in both arms (P=0.47).
The visual prognosis of study population was evaluated
also in means of visual gain and loss percentages at month 12. The ratio of
visual gain ≥3 Snellen lines (+15 ETDRS letters equivalent) at the final visit
was found in 1+PRN and 3+PRN arms as 11.9% and 15.6% (P=0.34),
respectively. In IVR subgroup analysis, the visual improvement (≥3 Snellen
lines) ratios in 1+PRN and 3+PRN arms revealed no significant difference (12% vs
13.8%, respectively; P=0.67). The same comparison in IVA group pointed
to a slight superiorty of 3+PRN (21.1%) over 1+PRN (11.5%) regimen, although it
did not reach a statistically significant level (P=0.27). The visual
loss ≥1 Snellen line (-5 ETDRS letters equivalent) was found in similar ratios
in 1+PRN and 3+PRN arms of the total study population (10.9% vs 11.9%,
respectively, P=0.96). Hence, In IVA and IVR subgroups both arms visual
loss analysis showed comparable ratios at month 12 (8.3% vs 6.6%, 13.3% vs
12.1%) respectively.
DISCUSSION
The AMD is one of the major etiologies for legal
blindness in the developed countries over a certain age and this status is
increasing exponentially with the overall life expectancy and crowding risk
factors. The mainstay therapy of this devastating disease remains still
anti-VEGF treatment options. Pivotal trials for administrative approvals
recommended several regimens such as fixed monthly, a PRN approach following
3-5 initial loading doses or a priori as needed regimen. All collective data
suggest a strict follow-up and prompt treatment since a delay of therapeutic
intervention might cause irreversible destruction of foveal microstructure
leading to a permanent visual impairment[9].
Despite this well-known fact, heavy treatment burden for both the patients and
clinicians inhibit an ideal therapeutic follow-up. Several real life based
studies[10-11] reported
already this deviation of the results in real settings from the data of
clinical trials conducted under controlled ideal circumstances. With the
current study we aimed to review our own real life nAMD treatment outcomes in
Turkish population and evaluate the effect of initial loading phase-based on
the data driving from the nine tertiary reference centers of the most populated
cities (Istanbul, Kocaeli) of Turkey.
Our anatomical results demonstrated no significant
difference in mean of CMT gains between 1+PRN and 3+PRN regimen arms in the
total study population and both-IVR or IVA-subgroups, although significantly
more intravitreal injections had been administrated in 3+PRN arms than in 1+PRN
arms of the total study population, IVR and IVA subgroups (4.4 vs 2.4;
4.67 vs 2.97; 4.2 vs 2.4, respectively). This finding might be
related to the fact that unlike the greater and continuous CMT reductions in e.g.
diabetic macular edema treatment outcomes, CMT value changes in nAMD remain
within a limited range due to the presence of the underlying and despite the
treatment persisting CNVM. Our mean CMT values remained almost unchanged after
the 6th month visit where all groups have obviously reached a
plateau in anatomical gain. We found this finding consistent with the previous
reports. In the two-year results of HARBOR study Ho et al[12] reported a rapid reduction of CMT at day 7 continuing
through month 3 and the CMT values sustained in the further 24mo follow-up to
the same extend regardless of treatment or dosing regimen. Although the most
frequent anatomical retreatment criterion was accepted as an increase in CMT[13], an analysis revealed the fact that CMT does not
correlate with visual function in AMD since this correlation between function
and structure is lost as early as month 3 of the follow-up[14].
In real life studies such as the LUMINOUS study[15], the average number of injections in year 1 was
reported as 4.3, 5.5, 4.7, and
The prospective single center PronTo study[21] advocated for the efficacy of an OCT based 3+PRN
ranibizumab regimen against the earlier recommended fixed monthly dosing approach.
In this study, Lalwani et al[21] reported
a visual gain of 11.1 letters comparable to ANCHOR and MARINA trials with a
significantly less mean number of injections (9.9 vs 24 each) in their
24mo follow-up. Previously, in the first year results of the same study, Fung et
al[22] reported a visual improvement of 9.3
letters (approximately 1.8 Snellen lines), a visual gain ≥15 letters in 35 % of
the patients and a mean CMT reduction of 178 µm compared to baseline. They
apparently achieved these results with a mean number of 5.6 injections at the
end of 12mo. These anatomical and functional outcomes demonstrate a clear
superiority over the results of our 3+PRN arm (178 vs 84 µm; 35% vs 15.6%).
We believe, the prospective PronTo studies’ strict monthly monitoring regimen
combined with “zero tolerance” retreatment criteria might contribute to this
significant difference. In contrast to their 12 monthly visits in one year, the
mean visit number in our 3+PRN arm was only 7.2, exposing our deficiency of
close follow-up in even a 3+PRN regimen.
There are several studies in the literature questioning
the necessity of the initial loading phase in anti-VEGF therapy of nAMD. Menon
et al[23] compared in their prospective
randomized BeMOc trial loading and no loading regimens of intravitreal 1.25 mg
bevacizumab and concluded that gain in visual outcome following a loading
regimen was not as impressive as expected but still clinically justified.
Additionally, the loading phase did not increase the first year’s injection number
significantly. Earlier reports also emphasized the importance of fixed initial
loading doses. Arias et al[24] found in a
non-randomized study with small sample size that a loading phase with
bevacizumab resulted in better visual outcome compared to no loading at the end
of 6mo follow-up. In a retrospective, non-randomized study Gupta et al[25] compared loading and non-loading IVR groups and
reported the superiority of their loading group over the non-loading in the
means of visual outcome. On the other hand, two studies claimed that a loading
phase might not be essential in the AMD treatment. In the CATT trial, the PRN
arms of both bevacizumab and ranibizumab groups had no initial loading phase
but the investigators found non-inferior final visual outcomes compared to the
fixed monthly regimen[26]. Later, El-Mollayess et
al[27] reported that there was no significant
difference in means of visual gain between a fixed monthly regimen of
bevacizumab and a priori PRN regimen without any loading doses. In both of
these studies there was apparently a strict follow-up and low threshold
retreatment protocol based on OCT findings. In our study population, however,
loading phase enhanced visual outcome in all subgroups significantly,
particularly due to the fact that we treated the patients in the 1+PRN groups
in a suboptimal dosing.
In conclusion, this retrospective study was the first
national broad-based nAMD research conducted by clinicians from nine most
referred clinical centers, reflecting the real life treatment results in
Turkish population. The limitations of this study such as clinician based
retreatment criteria on a PRN regimen or irregular visits were deriving from
its multi-centered and retrospective nature; we tried to eliminate these
limitations and selection bias by strictly following our exclusion-inclusion
and retreatment criteria and excluding the non-copying patient data from the
study. Following our first report[28], this
current study gave us all the investigators a critical insight into our
treatment preferences and its consequences within an earlier time interval.
Although both regimens resulted in similar anatomical outcomes in means of CMT,
the 3+PRN arm clearly demonstrated—regardless of the anti-VEGF agent-the vital
role of three initial consecutive doses for desirable visual outcomes. Hence,
all the investigators were convinced from the need of a re-adjustment of their
clinical approach and the importance of the initial loading phase in nAMD
treatment.
ACKNOWLEDGEMENTS
Authors’ contributions: Manuscript preparation: Erden B, Bölükbaşı S, Özkaya A;
Data collection: All the authors; Data evaluation: All the authors; Study
design and concept: Erden B, Bölükbaşı S, Özkaya A, Karabaş L.
Conflicts of Interest: Erden B, None; Bölükbaşı S, None; Özkaya
A, None; Karabaş L, None; Alagöz C, None; Alkın Z,
None; Artunay Ö, None; Bayramoğlu SE,None; Demir G, None; Demir
M, None; Demircan A, None; Erdoğan G, None; Erdoğan M,
None; Eriş E, None; Kaldırım H, None; Onur İU, None; Osmanbaşoğlu
ÖA, None; Özdoğan Erkul S, None; Öztürk M, None; Perente
İ, None; Sarıcı K, None; Sayın N, None; Yaşa D, None; Yılmaz
İ, None; Yılmazabdurrahmanoğlu Z, None; Bosphorus Retina Study
Group, None.
REFERENCES