
·Brief
Report·
Proteome
alterations in aqueous humour of primary open angle glaucoma patients
Hanhan
Liu1,2, Fabian Anders2, Sebastian Funke2, Karl
Mercieca3, Franz Grus2, Verena Prokosch1
1Department of Ophthalmology,
University Medical Center of the Johannes Gutenberg University Mainz, Mainz
55131, Germany
2Experimental and Translational
Ophthalmology, Department of Ophthalmology, University Medical Center of the
Johannes Gutenberg University Mainz, Mainz 55131, Germany
3Royal Eye Hospital, School of Medicine,
University of Manchester, Manchester M202UL, United Kingdom
Correspondence
to: Verena
Prokosch. University Eye Hospital Mainz, Johannes Gutenberg University of
Mainz, Langenbeckstrasse 1, Mainz 55131, Germany. vprokosch@gmx.de
Received:
Abstract
AIM: To
unravel the primary open angle glaucoma (POAG)
related proteomic changes in aqueous humour (AH).
METHODS: Totally
35 patients listed for cataract surgery (controls: n=12, age: 67.4±13.6y) or trabeculectomy
for POAG (n=23, age: 72.5±8.3y) were included. AH
samples of those patients were obtained during cataract surgery or
trabeculectomy. AH samples were subsequently pooled into the experimental
groups under equal contribution in terms of protein amount of each individual
patient. Protein samples were analyzed by a linear trap quadrupol Orbitrap Mass
Spectrometry device with an upstream liquid chromatography system. The obtained
raw data were analyzed using the Maxquant proteome software and compared.
Proteins with a fold-change ratio higher than a cut-off of 2 were considered as
noticeably altered.
RESULTS: A total number of 175
proteins could be identified out of the AH from POAG and cataract by means of
quantitative mass spectrometric analysis. Apolipoprotein D (fold change, 3.16
times), complement C3 (2.96), pigment epithelium-derived factor (2.86),
dickkopf-related protein 3 (2.18) and wingless-related integration (Wnt)
inhibitory factor 1 (2.35) were significantly upregulated within the AH of
glaucoma compared to cataract serving as controls.
CONCLUSION: AH provides a tool to
analyze changes in glaucoma and shows striking changes in Wnt signaling
inhibitory molecules and other proteins.
KEYWORDS: primary open angle
glaucoma; aqueous humor; proteomics; Wnt signaling pathway
DOI:10.18240/ijo.2020.01.24
Citation:
Liu H, Anders F, Funke S, Mercieca K, Grus F, Prokosch V. Proteome alterations
in aqueous humour of primary open angle glaucoma patients. Int J Ophthalmol
2020;13(1):176-179
INTRODUCTION
Glaucoma is
a leading cause of vision loss and blindness worldwide, characterized by
retinal ganglion cell (RGC) loss and axonal degeneration, resulting in
irreversible loss of vision. Elevated intraocular pressure (IOP) is one of the
main risk factors and until now the only treatable one. However, RGC loss
proceeds despite IOP control[1] and the pathogenesis remains still obscure. Loads
of investigational research has been done in the last decades to elucidate the
mechanisms taking place in glaucoma with nothing being ground-breaking.
Proteomics
provides a reasonable tool to look into the pathogenesis of a disease and ample
proteomic research has been done in animal models of glaucoma. However animal
models of glaucoma do not always reflect the disease state in humans and it is
thus needed to look into human tissue as well, which is difficult to get. The
aqueous humour (AH) is well-accessible. Besides this, its protein composition
alters depending on ocular diseases. Thus the change in AH protein may provide
an insight into involved molecular mechanisms of glaucoma and help us
understand the molecular changes in the context of the disease.
SUBJECTS AND METHODS
Ethical
Approval Informed
consent was obtained by the patients and the local Ethical Committee of
Rhineland Pfalz was asked for permission.
Patients
Selection Patients
that were listed for cataract surgery, who served as controls or for
trabeculectomy, who served as the primary open angle glaucoma (POAG) group in a
tertiary eye care centre in the second term of 2016 were included in the study.
AH samples from 35 patients were used for the investigation (controls: n=12,
age: 67.4±13.6y;
POAG: n=23, age: 72.5±8.3y). Each patient underwent a thorough ophthalmologic
examination including a review of medical history, best-corrected visual
acuity, slit-lamp biomicroscopy, IOP measurement, gonioscopy and dilated
fundoscopic examination. Patients were diagnosed with POAG when a reproducible
visual field defect or a reproducible deterioration in the appearance of the
optic disc was visible excluding any other reason for it, the angle was open
and no signs of pigment exfoliation or pseudoexfoliation were present. Exclusion
criteria were the following: 1) any ongoing ocular infection or within the
previous 3mo; 2) any onsite retinopathy or other retinal abnormalities; 3) any
onsite or history of ophthalmic trauma.
Sample
Collection Surgery was performed under local or
general anesthesia according to the special needs of each individual patient.
Samples of the AH were taken at the beginning of the cataract surgery or
trabeculectomy. After desinfection of the eyeball, a small
Quantitative Proteomic Measurements
and Software Assisted Proteomic Profiling
First of
all, the total protein amount of each sample was determined via
bicinchoninic acid protein assay. All samples were subsequently pooled in the
experimental groups. It was taken care that there was an equal contribution of
protein amount for each individual patient. The protein mixture was loaded onto
a 12% Bis-Tris gel and a sodium dodecyl sulfate polyacrylamide gel
electrophoresis was performed. After digestion, extraction and cleaning of the
generated peptides, the final measurement occurred in a linear trap quadrupol
Orbitrap Mass Spectrometry device with an upstream liquid chromatography
system. The obtained raw data were analyzed using the Maxquant proteome
software and compared.
Statistical Analysis Data were analyzed statistically
using the two-independent samples test (SPSS Statistica Version 7) for Gaussian
distributions, with the remaining quantitative data analyzed using two-way
analysis of variance (Statistica Version 7) with post-hoc analysis using the
Turkey HSD test to identify possible differences among the experimental groups.
If the distribution was not Gaussian, the Kruskal-Wallis H test was
used.
RESULTS
The age of the controls and POAG
patients were 67.4±13.6y and 72.5±8.3y, respectively. There was no significant
age difference. A total number of 175 proteins could be identified out of the
AH from POAG and cataract-patients by means of quantitative mass spectrometric
analysis. A couple of proteins showed a significant up-regulation in POAG
patients compared to the respective control cataract group. Those interesting
proteins were afamin (AFM; fold change 1.63, P<0.005), apolipoprotein
D (ApoD; fold change 3.16, P<0.005), complement C3 (C3; fold change
2.96, P<0.005), dickkopf-related protein 3 (DKK3; fold change 2.18, P<0.005),
wingless-related integration inhibitory factor 1 (WIF1; fold change 2.35, P<0.005),
pigment epithelium-derived factor (PEDF; fold change 2.86, P<0.005).
DISCUSSION
Proteomics
provides a reasonable tool to look into the pathogenesis of a disease and ample
proteomic research has been done in animal models of glaucoma. However animal
models of glaucoma do not always reflect the disease state in humans and it is
thus needed to look into human tissue as well, which is difficult to get. The
AH is well-accessible. Besides this, its protein composition alters depending
on ocular diseases. Thus the change in AH protein may provide an insight into
involved molecular mechanisms of glaucoma and help us understand the molecular
changes in the context of the disease.
Purpose of our study was to find
typical glaucoma-related protemic changes in the AH. We had the following
findings: We could identify 175 proteins in total. Among those AFM (fold change
1.63, P<0.005), ApoD (fold change 3.16, P<0.005), C3 (fold
change 2.96, P<0.005), DKK3 (fold change 2.18, P<0.005),
WIF1 (fold change 2.35, P<0.005), PEDF (fold change 2.86, P<0.005)
were significantly upregulated.
AFM is a pleiotropic glycoprotein
with neuroprotective properties in vitro, which might be related to binding
vitamin E acting as a radical scavenger[2] in
various neurodegenerative diseases[3-4].
ApoD is a small, soluble lipid
carrier. It is found in most human tissues, but mostly expressed in glia cells
of the central nervous system[5]. ApoD has been
described to play a role in various age-related and neurological disorders
including glaucoma[6]. A number of studies proved
ApoD’s ability to protect organisms and cells against both extrinsic and
intrinsic stress[6]. This could be partially
related to a direct scavenging activity against free radical damage[6]. As oxidative stress has been recognized as one of the
main pathogenetic factors in open angle glaucoma, AFM and ApoD might be
increased to inhibite oxidative and apoptotic damage in POAG patients.
C3 is a protein of the complement
system. The activated complement system clears cell and tissue debris. There is
accumulating knowledge that complement dysregulation is responsible for
numerous immune-mediated and inflammatory disorders. Imbalances in complement
regulation and oxidative stress may play a role as a risk factor contributing
to the dysregulation of complement activation in glaucoma[7].
Most interestingly a group of wingless-related integration (Wnt) signaling
pathway regulating proteins were observed and increased in POAG patients: DKK3,
WIF1 and PEDF (Figure 1).
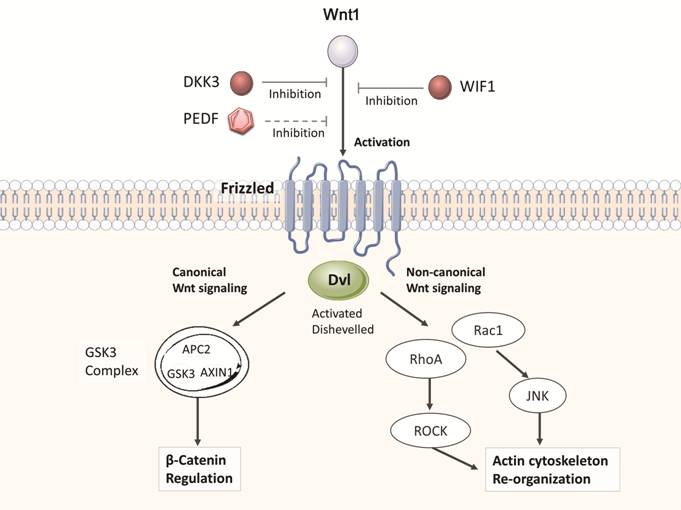
Figure 1 Overview of the chonical
and non-chonical Wnt-signaling pathways, generated with a pathway analysis
software Up-regulated aqueous humor proteins are
indicated in red and might have dramatic effects on Wnt-signaling in the
context of POAG. JNK: c-Jun N-terminal kinases; GSK3: Glycogen synthase
kinase-3; APC2: APC regulator of Wnt signaling pathway 2; Rac: Rho family of
GTPases.
Wnt comprises a diverse and
well-conserved family of secreted lipid-modified signaling glycoproteins that
are 350-400 amino acids in length. Wnts signaling is involved in plenty
processes in evolution, maturation and disease. The Wnt signaling pathways
maintain tissue homeostasis and regeneration[8],
promote axonal remodeling and synaptic differentiation[8],
and participate in the maturation, homeostasis and function of mature neurons.
Thus, regulation of Wnt signaling is crucial and can protect against
neurodegeneration[8]. Dickkopf (DKK) family and
WIF1 are known as Wnt signaling antagonists[9].
Among those Wnt signaling molecules
DKK family is a major class of Wnt signaling regulators. DKK3 is demonstrated
as an antagonist of Wnt signaling. There are studies indicating that DKK3 may
also play a protective role by inhibiting caspase activity in response to
retinal injury[10]. Unlike DKK1 and DKK2, its
role is yet not well studied in dysfunctional Wnt signaling.
PEDF is a multifunctional protein,
which plays a crucial role in various physiological and pathophysiological
conditions[11]. Studies show a significant
age-related decrease in PEDF levels[12]. It has
been seen in in vitro and in vivo that PEDF can inhibit RGC
apoptosis exerting potential neuroprotective features[13].
In addition to this, PEDF has been recognized as a novel Wnt pathway antagonist[13].
Wnt activity plays a positive role
in neurodegeneration and regulation of IOP. In our study, three Wnt pathway
antagonists, PEDF, DKK3 and WIF1 were found up-regulated in POAG patients,
indicating a possible role of Wnt signaling in the pathophysiology of glaucoma.
Whether Wnt pathway is involved in neurodegeneration and/or regulation of IOP
is still unclear and requires further study.
In correlation with our findings,
AFM, ApoD, DKK3 and PEDF were found up-regulated in the AH of POAG patients
after implantation of a shunt device[14-16]
backing our findings. Thus exploring Wnt signaling in glaucoma patients more in
detail might provide some new prospective for further studies.
In conclusion, the AH provides a
tool to analyze and possibly better understand the pathophysiology of glaucoma.
We could find striking changes in Wnt signaling inhibitory molecules and other
proteins, which are known for their importance in neurodegenerative conditions.
This might help to understand and diagnose the disease much better in the
future and find novel treatments[17-20].
ACKNOWLEDGEMENTS
Foundation: Suppored by German Research
Foundation (DFG 1569 1-1).
Conflicts of Interest: Liu H, None; Anders F, None; Funke
S, None; Mercieca K, None; Grus F, None; Prokosch V, None.
REFERENCES
|
1 Bagnis A, Papadia M, Scotto R, Traverso CE. Current
and emerging medical therapies in the treatment of glaucoma. Expert Opin
Emerg Drugs 2011;16(2):293-307. |
|
|
|
|
|
2 Altamirano A, Naschberger A, Fürnrohr BG, et al.
Expression, purification, and biochemical characterization of human afamin. J
Proteome Res 2018;17(3):1269-1277. |
|
|
|
|
|
3 Ringman JM, Schulman H, Becker C, et al. Proteomic
changes in cerebrospinal fluid of presymptomatic and affected persons
carrying familial Alzheimer disease mutations. Arch Neurol 2012;69(1):96-104. |
|
|
|
|
|
4 Rosenling T, Stoop MP, Attali A, van Aken H,
Suidgeest E, Christin C, Stingl C, Suits F, Horvatovich P, Hintzen RQ,
Tuinstra T, Bischoff R, Luider TM. Profiling and identification of
cerebrospinal fluid proteins in a rat EAE model of multiple sclerosis. J
Proteome Res 2012;11(4):2048-2060. |
|
|
|
|
|
5 Muffat J, Walker DW. Apolipoprotein D: an overview of
its role in aging and age-related diseases. Cell Cycle 2010;9(2):269-273. |
|
|
|
|
|
6 Son JH, Chung YK, Son JS. Apolipoprotein B: novel
indicator of elevated intraocular pressure. Eye (Lond) 2015;29(10):1315-1320. |
|
|
|
|
|
7 Clark SJ, Bishop PN. The eye as a complement
dysregulation hotspot. Semin Immunopathol 2018;40(1):65-74. |
|
|
|
|
|
8 Webber HC, Bermudez JY, Millar JC, Mao W, Clark AF.
The role of Wnt/β-Catenin signaling and K-Cadherin in the pathogenesis of
glaucoma. Invest Ophthalmol Vis Sci 2018;59(3):1454-1466. |
|
|
|
|
|
9 Fragoso MA, Yi H, Nakamura RE, Hackam AS. The Wnt
signaling pathway protects retinal ganglion cell 5 (RGC-5) cells from
elevated pressure. Cell Mol Neurobiol 2011;31(1):163-173. |
|
|
|
|
|
10 Mao W, Millar JC, Wang WH, Silverman SM, Liu Y,
Wordinger RJ, Rubin JS, Pang IH, Clark AF. Existence of the canonical Wnt
signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci
2012;53(11):7043-7051. |
|
|
|
|
|
11 Lee SJ, Duncan DS, Echevarria FD, McLaughlin WM,
Hatcher JB, Sappington RM. Pressure-induced alterations in PEDF and PEDF-R
expression: implications for neuroprotective signaling in glaucoma. J Clin
Exp Ophthalmol 2015;6(5):491. |
|
|
|
|
|
12 Park K, Lee K, Zhang B, Zhou T, He X, Gao G, Murray
AR, Ma JX. Identification of a novel inhibitor of the canonical Wnt pathway.
Mol Cell Biol 2011;31(14):3038-3051. |
|
|
|
|
|
13 Olafsdottir OB, Hardarson SH, Gottfredsdottir MS,
Harris A, Stefánsson E. Retinal oximetry in primary open-angle glaucoma.
Invest Ophthalmol Vis Sci 2011;52(9):6409-6413. |
|
|
|
|
|
14 Anshu A, Price MO, Richardson MR, Segu ZM, Lai X,
Yoder MC, Price FW Jr. Alterations in the aqueous humor proteome in patients
with a glaucoma shunt device. Mol Vis 2011;17:1891-1900. |
|
|
|
|
|
15 Guo T, Guo L, Fan Y, Fang L, Wei J, Tan Y, Chen Y,
Fan X. Aqueous humor levels of TGFβ2 and SFRP1 in different types of
glaucoma. BMC Ophthalmol 2019;19(1):170. |
|
|
|
|
|
16 Kaur I, Kaur J, Sooraj K, Goswami S, Saxena R,
Chauhan VS, Sihota R. Comparative evaluation of the aqueous humor proteome of
primary angle closure and primary open angle glaucomas and age-related
cataract eyes. Int Ophthalmol 2019;39(1):69-104. |
|
|
|
|
|
17 Adav SS, Wei J, Qian J, Gan NY, Yip LWL, Sze SK.
Aqueous humor protein dysregulation in primary angle-closure glaucoma. Int
Ophthalmol 2019;39(4):861-871. |
|
|
|
|
|
18 Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath
V. Review of biomarkers in ocular matrices: challenges and opportunities.
Pharm Res 2019;36(3):40. |
|
|
|
|
|
19 Millar JC, Savinainen A, Josiah S, Pang IH. Effects
of TAK-639, a novel topical C-type natriuretic peptide analog, on intraocular
pressure and aqueous humor dynamics in mice. Exp Eye Res 2019;188:107763. |
|
|
|
|
|
20 Bauer D, Kasper M, Walscheid K, Koch JM, Müther PS,
Kirchhof B, Heiligenhaus A, Heinz C. Alteration of MCP-1 and MMP-9 in aqueous
humor is associated with secondary glaucoma in Fuchs uveitis syndrome. Ocular
Immunol Inflamm 2019:1-11. |
|