
·Letter
to the Editor·
Primary
conjunctival tuberculosis in two middle-aged women
Zhi-Qiao
Liang1, Qin Zhang1, Ming-Wei Zhao1, Xiao-Xin
Li2, Ming-Wu Li3
1Department of Ophthalmology, Peking
University People’s Hospital, Beijing 100044, China
2Department of Ophthalmology, Xiamen
Eye Center of Xiamen University, Xiamen 361003, Fujian Province, China
3Department of Ophthalmology, Peking
University International Hospital, Beijing 102206, China
Correspondence to: Ming-Wu Li. Department of
Ophthalmology, Peking University International Hospital, Shengmingyuan Street 1st,
Changping District, Beijing 102206, China. drlmwlmw@163.com
Received:
DOI:10.18240/ijo.2020.01.25
Citation: Liang
ZQ, Zhang Q, Zhao MW, Li XX, Li MW. Primary conjunctival tuberculosis in
two middle-aged women. Int J Ophthalmol 2020;13(1):180-183
Dear Editor,
We are writing to present two case
reports on primary conjunctival tuberculosis (TB) in two middle-aged women. TB
is an airborne communicable disease and is identified as the second leading
cause of death from infectious disease worldwide[1].
TB primarily affects the lung, but may also affects extrapulmonary organs,
including the eye[2]. Ocular TB consists of a
group of manifestations caused by the acid-fast bacillus M. Tuberculosis. The
first conjunctival TB case was recorded by Koaster in 1873[3].
At present, this entity is a rare condition and the standards of diagnosis and
treatment have yet to be well-established. The aim of this study was to report
two cases of primary conjunctival TB in two middle-aged women who were
presented with swollen eyelids and foreign body sensation.
CASE REPORT
Case
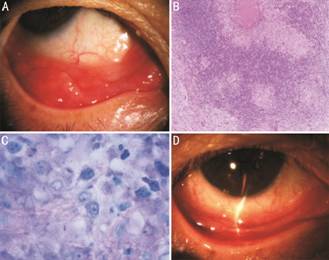
Figure 1 Case
She received mass excision
operation. Under topical anesthesia with benoxinate hydro chloride (20 mL:80
mg) and local anesthesia with lidocaine (2%), the mass was excised and sent for
histopathological examination. We recommended that she use ofloxacin eye
ointment (
Then she was taken some further
investigations. Purified protein derivative (PPD) placement produced a strongly
positive test (16×
Case
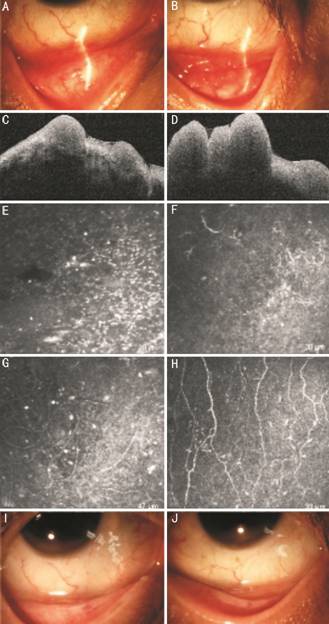
Figure 2 Case
In addition to these, she underwent
anterior segmental optical coherence tomography (AS-OCT) and in vivo
confocal microscopy (IVCM). AS-OCT revealed plenty of solid masses in both
fornix conjunctiva (Figure
DISCUSSION
TB is identified as a severe global
health problem, which causes millions of people in poor health condition each
year and ranks among the leading cause of fatality worldwide[1].
In 2014, the World Health Assembly ambitiously published their target to
eradicate TB as a public health threat by 2035[5].
TB primarily affects the lung.
Despite this, it has also been found to damage other organs, including the eye.
TB affects the eye through direct infection of the tubercle bacillus or via
a hypersensitivity reaction to the bacillus located elsewhere in the body.
Ocular lesions resulting from M. Tuberculosis are diverse and are capable of
impairing any structure of the eye and adnexa[6].
Conjunctival TB sites frequently
involved subconjunctival tissue[7], bulber
conjunctiva[8-11] and tarsal
conjunctiva[12-13].
Infrequently, they may arise in fornix conjunctiva[14-15], as in our cases. Nevertheless, we suggest that the
actual incidence is significantly higher than expected as it will be easily
overlooked both by patients and by doctors.
The immunoallergic state of the
patient could lead to the different kinds of clinical manifestations[14]. Based on the observation made of 160 cases of
conjunctival TB, Eyre (1912) divided the morphological characteristics of
conjunctival lesion into 4 categories[3]:
ulcerative, nodular, hypertrophic granulomatous and pedunculated. Our patient
showed the signs of hypertrophic granulomatous type. Other contributory factors
in causing granulomatous inflammations of conjunctiva must be kept in mind,
such as foreign-body granulomas, sarcoidosis, parinaud ocular glandular
syndrome and syphilis[16]. In our cases, there
was no history or microscopic evidence of any foreign body. In the first case,
the granulomas were necrotized, surrounded with epithelioid histiocytes and
rimmed with lymphocytes, which was found to be inconsistent with the
characteristics associated with sarcoidosis. In the second case,
angiotensin-converting enzyme level was within normal limits and no significant
abnormality were detected from CXR, thus ruling out the possibility of sarcoidosis.
The lab investigations of both patients were all within normal limits including
treponema pallidum haemagglutination.
In case 1, the initial clinical
presentation was suggestive of conjunctival MALT. However, the conjunctival
histologic features of necrotizing granulomatous inflammation and positive
Ziehl-Neelsen acid-fast stain result pointed to the diagnosis of TB. Due to the
lack of pulmonary specific lesions, a diagnosis of primary conjunctival TB was
made. Not only can the conjunctival reaction arise from contact with a
contaminated finger or tissue[14], it can also be
caused by inappropriate response of activated T cells left exposed to
tubercular antigen in the lymph nodes and subsequently migrated to the
conjunctival surface[9].
In case 2, we applied AS-OCT and
IVCM to explore the characteristics of the fornix conjunctival masses. AS-OCT
is viewed as a reliable tool to measure the cross-sectional area of corneal and
anterior segment diseases, especially conjunctival diseases, like conjunctivochalasis[17], pterygium, pinguecula[18],
melanoma, nevi[19-20] and
conjunctival lymphoma[19]. In our case, the
AS-OCT findings revealed nothing abnormal in respect to epithelial appearance
and thickness. A sub-epithelial mass was identified as being hyporeflective
and there was shadowing of the underlying tissue. Instead of follicle-like
masses, it was confirmed as solid masses that was quite contrary to the results
of our slit lamp examination.
IVCM is a novel, non-invasive
technique that is capable of providing high-resolution images of living tissues
and can be accurate to the cellular level[21-22]. The illumination and observation systems need to
focus on the same focal point, which is the optical principle of this novel
technique[23]. It has been applied to performing
study on a wide range of infectious, especially in diagnosing of microbial
keratitis, where it may assist with the identification of filamentary fungi and
acanthamoeba cysts[22-23].
To the best of our knowledge, this
was the first time IVCM has been reported from individuals with conjunctival
TB. There are evidences suggesting both conjunctiva and cornea are in the
inflammatory state. Not only could we find much more subepithelial round cell
infiltrate and cells with multilobate nucleus than normal conjunctiva, we could
also discover many Langerhans cells in the subepithelial layer of cornea.
Despite this, we have yet to observe any special structure from IVCM in
comparison with classic histopathology of biopsy specimens.
To conclude, making the diagnosis of
conjunctival TB can be challenging as there are a variety of manifestations and
the diagnostic criteria is not uniform at a global scale.The diagnosis of
conjunctival TB should be kept in mind when the patients exhibiting symptoms of
chronic conjunctivitis, swollen eyelids or foreign body sensation without
significant improvement by taking conservative treatments. Palpable
preauricular or other regional lymphadenopathy may provide a clue to the
diagnosis of conjunctival TB. Once conjunctival TB is being considered, further
diagnostic testing needs to be performed, including tuberculin skin testing and
interferon-gamma release assay, though they are not absolute reliable because
of the false positive and false negative possibilities. The gold standard of
diagnosis of conjunctival TB is biopsy. Non-invasive methods, including AS-OCT
and IVCM, are recognized as the reliable tools to examine the cross-sectional
area of conjunctival masses. Maybe in the future, with more studies on AS-OCT
and IVCM of conjunctival diseases, we will have more alternative choices to
diagnose and monitor this entity.
ACKNOWLEDGEMENTS
Authors’ contributions: Liang ZQ and Zhang Q recruited the
patients. Liang ZQ wrote the manuscript. Li MW, Li XX, and Zhao MW reviewed the
manuscript. All authors have approved the manuscript.
Conflicts of Interest: Liang ZQ, None; Zhang Q,
None; Zhao MW, None; Li XX, None; Li MW, None.
REFERENCES
|
1 Global tuberculosis report 2015. World Health
Orgnization 2015. http://www.who.int/entity/tb/publications/global_report/en/index.html. |
|
|
|
|
|
2 Gupta V, Shoughy SS, Mahajan S, Khairallah M,
Rosenbaum JT, Curi A, Tabbara KF. Clinics of ocular tuberculosis. Ocul
Immunol Inflamm 2015;23(1):14-24. |
|
|
|
|
|
3 Hadj MKK, Hadrich M, Bouslah H, Ben Abdelaziz A,
Charrada A, Aissa W. The hunterian lecture on tuberculosis of the
conjunctiva: its etiology, pathology, and diagnosis. Lancet
1912;179(4629):1319-1328. |
|
|
|
|
|
4 Helm CJ, Holland GN. Ocular tuberculosis. Surv
Ophthalmol 1993;38(3):229-256. |
|
|
|
|
|
5 Zumla A, George A, Sharma V, Herbert RHN, Oxley A,
Oliver M. The WHO 2014 Global tuberculosis report-further to go. Lancet Glob
Heal 2015;3(1):e10-e12. |
|
|
|
|
|
6 Alvarez GG, Roth VR, Hodge W. Ocular tuberculosis:
diagnostic and treatment challenges. Int J Infect Dis 2009;13(4):432-435. |
|
|
|
|
|
7 Biswas J, Kumar SK, Rupauliha P, Misra S, Bharadwaj
I, Therese L. Detection of Mycobacterium tuberculosis by nested polymerase
chain reaction in a case of subconjunctival tuberculosis. Cornea
2002;21(1):123-125. |
|
|
|
|
|
8 Feinstein E, Farooq AV, Lin AY, Traish AS. Bilateral
conjunctival tuberculosis presenting as mass lesions. Can J Ophthalmol
2015;50(1): e11-e13. |
|
|
|
|
|
9 Sharma K, Kalakoti P, Juneja R, Sahu, Singh V,
Subramanian PS. Re-emphasizing Thygeson's warning: conjunctival phlyctenulosis
as presenting sign of impending clinical tuberculosis. Can J Ophthalmol
2014;49(6):e135-e137. |
|
|
|
|
|
10 Libby GF. Tuberculosis of the bulbar conjunctiva.
Trans Am Ophthalmol Soc 1914;13(Pt 3):784-786.1. |
|
|
|
|
|
11 Browning SH. Tuberculosis of conjunctiva. Proc R Soc
Med 1943;36(8):407-408. |
|
|
|
|
|
12 Rose JS, Arthur A, Raju R, Thomas M. Primary
conjunctival tuberculosis in a 14 year old girl. Indian J Tuberc
2011;58(1):32-34. |
|
|
|
|
|
13 Brar RK, Singh A, Deshpande AH, Gargade CB, Das S.
Primary conjunctival tuberculosis presenting as dry eye: a rare case report
and review of the literature. Ocul Oncol Pathol 2017;3(4):276-278. |
|
|
|
|
|
14 Sollom AW. Primary conjunctival tuberculosis. Br J
Ophthalmol 1967;51(10):685-687. |
|
|
|
|
|
15 Solmaz N, Önder F, Demir N, Altuntaş Aydın Ö.
Primary conjunctival tuberculosis. Turk J Ophthalmol 2018;48(1):39-41. |
|
|
|
|
|
16 Fernandes M, Vemuganti GK, Pasricha G, Bansal AK,
Sangwan VS. Unilateral tuberculous conjunctivitis with tarsal necrosis. Arch
Ophthalmol 2003;121(10):1475-1478. |
|
|
|
|
|
17 Gumus K, Crockett CH, Pflugfelder SC. Anterior
segment optical coherence tomography: a diagnostic instrument for
conjunctivochalasis. Am J Ophthalmol 2010;150(6):798-806. |
|
|
|
|
|
18 Soliman W, Mohamed TA. Spectral domain anterior
segment optical coherence tomography assessment of pterygium and pinguecula.
Acta Ophthalmol 2012;90(5):461-465. |
|
|
|
|
|
19 Nanji AA, Sayyad FE, Galor A, Dubovy S, Karp CL.
High-resolution optical coherence tomography as an adjunctive tool in the
diagnosis of corneal and conjunctival pathology. Ocul Surf
2015;13(3):226-235. |
|
|
|
|
|
20 Thomas BJ, Galor A, Nanji AA, El Sayyad F, Wang JH,
Dubovy SR, Joag MG, Karp CL. Ultra high-resolution anterior segment optical
coherence tomography in the diagnosis and management of ocular surface
squamous neoplasia. Ocul Surf 2014;12(1):46-58. |
|
|
|
|
|
21 Long Q, Zuo YG, Yang X, Gao TT, Liu J, Li Y.
Clinical features and in vivo confocal microscopy assessment in 12 patients
with ocular cicatricial pemphigoid. Int J Ophthalmol 2016;9(5):730-737. |
|
|
|
|
|
22 Hu VH, Weiss HA, Massae P, Courtright P, Makupa W,
Mabey DC, Bailey RL, Burton MJ. In vivo confocal microscopy in scarring
trachoma. Ophthalmology 2011;118(11):2138-2146. |
|
|
|
|
|
23 Hu VH, Holland MJ, Cree IA, Pullin J, Weiss HA,
Massae P, Makupa W, Mabey DC, Bailey RL, Burton MJ, Luthert P. In vivo
confocal microscopy and histopathology of the conjunctiva in trachomatous
scarring and normal tissue: a systematic comparison. Br J Ophthalmol
2013;97(10):1333-1337. |
|
|
|
|